Insidious and Deadly
History and Course of Illness
An 89-year-old woman presented to her primary care physician with nonspecific symptoms, including weight loss, fatigue, and mild back pain. Occasional “itchiness” and skin irritation were becoming more frequent and particularly bothersome. Blood tests revealed elevated liver function tests, notably bilirubin (conjugated and total) and alkaline phosphatase. Relevant history included mild to moderate cardiovascular disease and smoking over the course of several decades. A contrast-enhanced CT scan of the abdomen was performed, followed by MR cholangiopancreatography (MRCP).
Findings
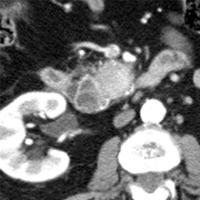
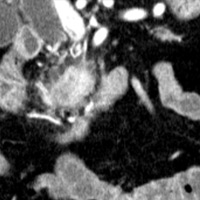
Contrast-enhanced CT scan of the abdomen demonstrated dilatation of the common bile duct (CBD) up to 2.1 cm, with an abrupt cutoff at the level of the pancreatic head. Biliary dilatation extended cephalad, involving much of the central bile ducts. There was enlargement of the pancreatic head, which contained an ill-defined region of relative hypoattenuation. The pancreatic body and tail were not visualized compatible with marked fatty atrophy. A few scattered para-aortic nodes were present, with most less than 1 cm in short axis. No vascular encasement, vessel in-growth, or hepatic lesions were identified. MRI demonstrated a relatively hypoenhancing poorly defined mass surrounded by scant enhancing parenchyma. T2-weighted MR cholangiographic images vividly displayed biliary dilatation.
Diagnosis
Pancreatic adenocarcinoma (History, clinical, and radiologic findings are virtually diagnostic.)
Discussion
Pancreatic adenocarcinoma is a lethal malignancy. Only total surgical resection is considered curative, with fewer than 20% of patients deemed operable candidates. (An even smaller percentage undergo radical resection.) Unfortunately, most patients have significant tumor burden with vascular encasement and/or metastatic disease at initial presentation. Older patients are affected disproportionately, with a slight male predilection. Incidence is comparatively lower than several other solid-organ malignancies; however, pancreatic carcinoma is by no means a rare tumor. The insidious growth pattern of this neoplasm contributes to its unfavorable prognosis, with most patients suffering from painless jaundice at the time of initial imaging.1
Anatomically, the majority of adenocarcinomas arise within the pancreatic head. Ultrasound is not the imaging of choice for deciding on operative management but is reported to have reasonable sensitivity and specificity for detecting pancreatic carcinoma. Usually, the most striking abnormality is profound dilatation of the CBD. There may be accompanying dilatation of the intrahepatic biliary tree. Dilatation of both the CBD and the main pancreatic duct (Wirsung) is commonly known to radiologists as the “double-duct sign.” Aside from excluding benign causes of obstruction (eg, stones, inflammatory strictures), a pancreatic head lesion must be ruled out. In clinical practice, abrupt CBD dilatation in an older patient should prompt further imaging to exclude a pancreatic neoplasm even if pancreatic ultrasound is unremarkable. When a lesion is visualized, a hypoechoic mass replacing or located within the head of the pancreas is relatively diagnostic.2
CT is often the primary imaging study and, in the majority of cases, sufficient to verify the presence of a mass, determine the level/degree of obstruction, and assess specific variables that are surgically relevant—namely, degree of vascular encasement, nodal metastasis, and/or distant metastatic disease. Pancreatic adenocarcinoma is a hypovascular tumor, generally appearing as a hypoattenuating mass on contrast-enhanced CT, particularly during the arterial phase. Dedicated pancreatic protocols are designed to enhance lesion visualization and characterization. Exact margins of the tumor can be difficult to discern, and measurements of tumor size are often imprecise. Some tumors may be isodense to pancreatic parenchyma precluding CT localization or just too small to characterize. In such cases, findings such as the double-duct sign, with abrupt termination of the CBD and/or marked atrophy of the body/tail, are fairly specific.
MRI of the pancreas can be performed as an adjunctive examination, particularly for smaller lesions. Adenocarcinoma appears as a relatively hypointense mass compared with normal high-signal-intensity pancreatic parenchyma on T1 gradient echo images. Similar to contrast-enhanced CT, the tumor is visualized as a non- or hypoenhancing mass against a background of normally enhancing pancreatic tissue. Evaluation of the biliary tree and pancreatic duct is typically performed by MRCP.3
In addition to making (or suggesting) the diagnosis, a critical role for the radiologist is to determine resectability. What makes pancreatic adenocarcinoma unresectable? Essentially tumor in-growth into specific organs (eg, stomach, colon, mesocolon), hepatic and/or peritoneal/mesenteric/para-aortic nodal metastasis, vascular encasement greater than 180 degrees, and/or in-growth of the celiac axis, hepatic artery, or superior mesenteric artery.4
Is there a differential diagnosis for pancreatic head adenocarcinoma? Sure, and potential mimickers include lymphoma, metastatic disease, and even focal pancreatitis. However, a benign etiology, such as focal inflammation, is highly unlikely if many of the associated imaging findings aforementioned are present. In fact, if there is sufficient clinical and radiologic evidence for pancreatic adenocarcinoma, prompt surgical consultation and management should be the next step.5
— Rahul V. Pawar, MD, DABR, is an attending neuroradiologist at Saint Barnabus Medical Center in Livingston, N.J
 |
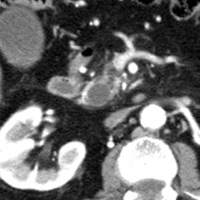 |
| Figure 1 (axial) — Enlarged enhancing pancreatic head with infiltrative region of relative hypoenhancement anteriorly | Figure 2 (axial) — Common bile duct (CBD) dilatation |
 |
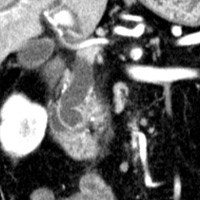 |
| Figure 3 (coronal) — Pancreatic head enlargement and associated hypovascular mass (proximally, CBD dilatation is evident) | Figure 4 (coronal) — Abrupt cutoff of the CBD at the level of the pancreatic head (“malignant tapering”); remainder of body/tail not seen (distal atrophy) |
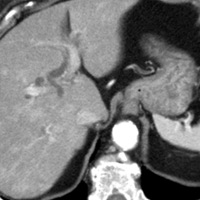 |
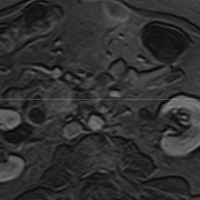 |
| Figure 5 (axial) — Central intra-hepatic biliary dilatation | Figure 6 (axial, postcontrast T1WI) — Enlarged pancreatic head with ill-defined region of hypoenhancement within (exact margins of tumor, unclear) remainder of pancreas not seen (body/tail atrophy) |
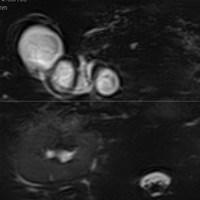 |
|
| Figure 7 (MRCP, axial T2WI) — CBD dilatation (2.1 cm) |
REFERENCES
- Di Magno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association. Gastroenterol. 1999;117(6):1464-1484.
- Karlson BM, Ekbom A, Lindgren PG, Kallskog V, Rastad J. Abdominal US for diagnosis of pancreatic tumor: Prospective cohort analysis. Radiology. 1999;213(1):107-111.
- Manoharan P, Sheridan MB. Neoplasms of the pancreas. Imaging. 2004;16(4):323-337.
- O’Malley ME, Boland GW, Wood BJ, Fernandez-del Castillo C, Warshaw AL, Mueller PR. Adenocarcinoma of the head of the pancreas: Determination of surgical unresectability with thin-section pancreatic-phase helical CT. Am J Roentgenol. 1999;173(6):1513-1518.
- van Gulik TM, Moojen TM, van Geenen R, Rauws EA, Obertop H, Gouma DJ. Differential diagnosis of focal pancreatitis and pancreatic cancer. Ann Oncol. 1999;10(Suppl 4):85-88.
ON THE CASE submission requirements
- Cases should have clinical relevance and clear radiological findings.
- Sections should include a title, history and course of illness, findings, diagnosis, and discussion.
- Maximum word limit should not exceed 800. At least three references are recommended.
- Cases may be submitted from any radiological subspecialty and imaging modality.
- Figures must be high-quality JPEG or TIFF images and labeled for ease of reference. Please keep images in their native format, without the addition of arrows or other means of highlighting the key findings.