Web Exclusive
Cryogen-Free MRI for Preclinical Research
BY DAVID TAYLOR, PHD
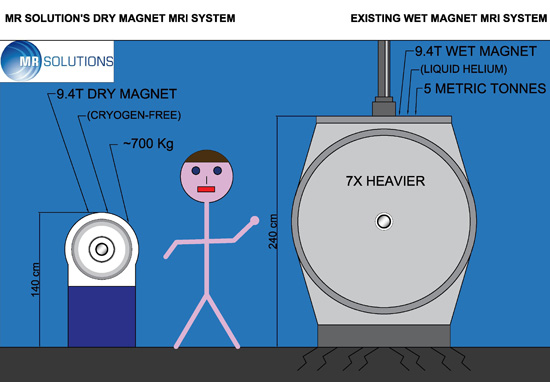
Preclinical imaging is undergoing radical change as researchers have greater access to a broader selection of imaging technologies than ever before. One of these technologies, cryogen-free MRI, is gaining popularity. Commercially available for only a few years, cryogen-free MRI scanners use a dry magnet system that does not require liquid helium, making them a fraction of the size and weight of traditional MRI scanners. They can be easily accommodated inside a lab, and they no longer interfere with other sensitive equipment because their stray magnetic field is only a few centimeters wide. In addition, cryogen-free MRI scanners have the ability to incorporate multimodality imaging capability and, most importantly, provide improved image resolution.
Researchers conduct preclinical imaging to visualize living animals at the molecular level for drug development purposes, visualization of disease progression, and other medical research. Multimodality imaging systems for preclinical research can provide MRI imaging with PET and SPECT imaging accessories. This allows MRI imaging to be carried out with PET or SPECT or as standalone devices.
Background to Preclinical MRI
To achieve high-resolution scans, the magnet strength, or Teslas (Ts), in MRI scanners must be high field. To achieve high fields, the coils of the magnet must carry high current. This can only be achieved using superconducting wire, which must be kept at or below 4° Kelvin (-452.47° F).
Traditionally, this very low temperature was achieved by immersing the magnet coils in a bath of liquid helium. This results in a very large, heavy, and expensive scanner—many tons—requiring its own special room and often considerable and expensive building adaptations. In addition, provision has to be made for emergency venting in the event of the magnet "quenching," ie, the liquid helium turning into gas unexpectedly, which otherwise could asphyxiate those using the scanner. Overall, this customization could increase the cost of the system by hundreds of thousands of dollars.
Cryogen-Free MRI Technology
Cryogen-free MRI replaces the liquid helium jacket with a revolutionary magnet design incorporating superconducting magnet coils that are cooled by direct conduction from a readily available, off-the-shelf cryocooler refrigerator unit. This results in a much lighter system—with a magnet weight, depending on system size, of approximately 350 kg (772 lbs), compared with two tons previously—which can be wheeled through the door into an ordinary laboratory with no special site alterations. It also allows for a shield coil to be placed optimally within the magnet to reduce the stray magnetic field from meters to centimeters.
This redesign of the magnet and the resulting MRI systems took more than three years with many iterations. The range has grown with models at 3 T, 4.7 T, 7 T, and 9.4 T, with a range of bore sizes in each model. All of the scanners can be combined with either simultaneous or in-series PET and/or SPECT capabilities.
Other benefits of not having the magnet in a liquid helium bath include the following:
• the operator can change the T power of the magnet, which is very important in research using reagents; and
• the scanners can be used in class 2 and 3 laboratories because there is no exhaust requirement and, with sealed animal cradles, the integrity of the lab is maintained.
Multi-Imaging Capability
Cryogen-free MRI is a multimodality system. SPECT and PET imaging modalities are some of the most suitable modalities for small animal in-vivo imaging.
To provide SPECT images, the four gamma camera heads and focusing collimator can be easily clipped on to the front of the bore of the MRI scanner to provide state-of-the-art 3D SPECT images. The SPECT images can be registered with the MRI images, providing anatomical-functional combined capability. The SPECT gamma camera can also be used independently.
The PET capability is provided by new solid-state detectors that are incorporated in the bore of the MRI scanner. The technology combines the structural and functional characterization of tissue provided by MRI with the sensitivity of PET imaging for viewing metabolism and tracking uniquely labeled cell types or cell receptors.
How It Works
To acquire an image, the researcher selects a software sequence that enables the imaging of the appropriate type of tissue, organ, or function. These sequences are all specific to such imaging, having been tried and tested. Sequences are always being written and rewritten to enable more detailed and specific targeted studies.
The sequence switches the gradient magnetic field on and off, allowing the imaging software to build images of the area of interest. These can be slices on different planes and axes to allow detailed viewing of the animal's anatomy. The actual sequence time can be minutes or hours, but typically the former, thus allowing many image acquisitions in the animal's resting state, often up to an hour or more.
The acquired images can be examined and exactly the same study can be carried out at a later date to track any change. The platform is not just restricted to animals; it can also be used for rock cores (eg, for the oil industry), plants, foodstuffs, and other porous substances. Often, contrast agents which allow enhanced viewing of functions and circulation within the body are injected into the live species, aiding the detection of changes and movement essential to research.
Preclinical imaging is of particular benefit to the advancement of groundbreaking medicines, treatments, and quantification tools for drug discovery and development. Scanning technologies are increasingly being applied to developmental toxicology studies in drug development to determine potential compound toxicity. This can begin to speed up drug development research to help pharmaceutical companies bring the latest drugs to market faster. Although most of these studies are conducted in nonregulatory settings, there is interest in performing these imaging studies under applicable regulations, eg, Good Laboratory Practices, to support regulatory decisions concerning drug safety. The use of cryogen-free, multimodality imaging technologies for translational research applications is moving forward with new developments all the time; this is an exciting time for the preclinical imaging market with significant market growth expected over the coming years.
— David Taylor, PhD, is CEO of MR Solutions.

