 On the Case
On the Case
By Pramod Gupta, MD, and Robert Sims, MD
Radiology Today
Vol. 22 No. 3 P. 30
History
A 69-year-old man with a history of chronic alcohol-induced pancreatitis presented to the emergency department (ED) with dyspnea, intractable vomiting, and abdominal pain. A nasogastric tube was placed, and a chest radiograph obtained. Based on the chest X-ray findings, the left pleural space was tapped for symptomatic relief and the patient was admitted for further imaging and management.
Findings
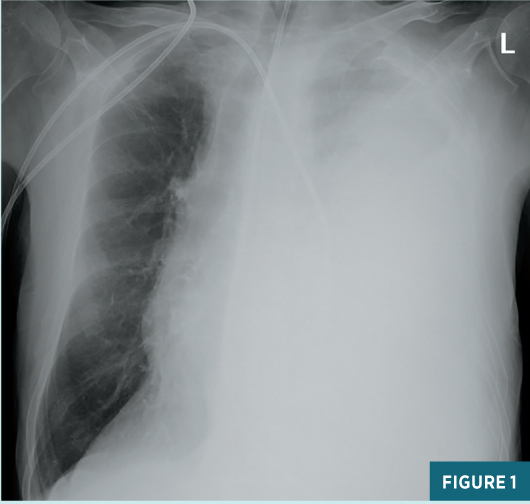
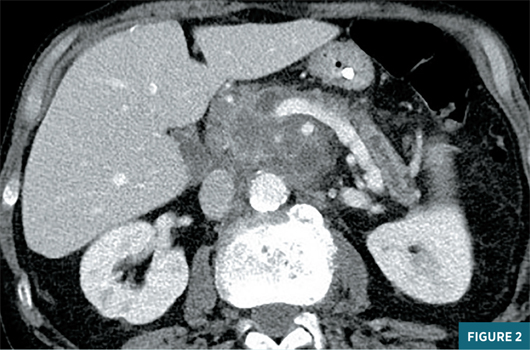
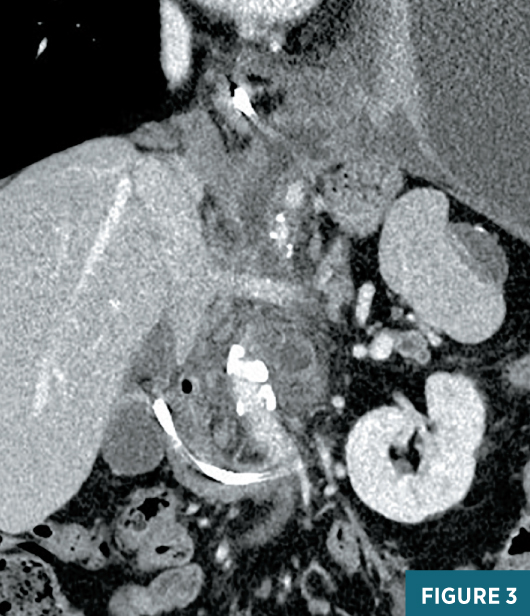
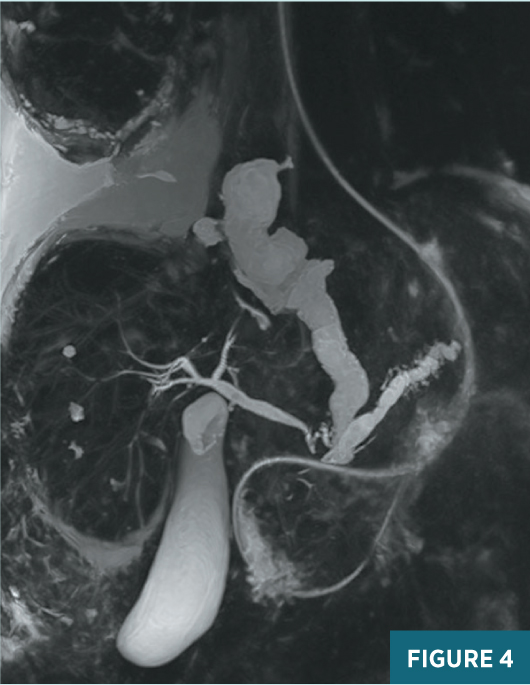
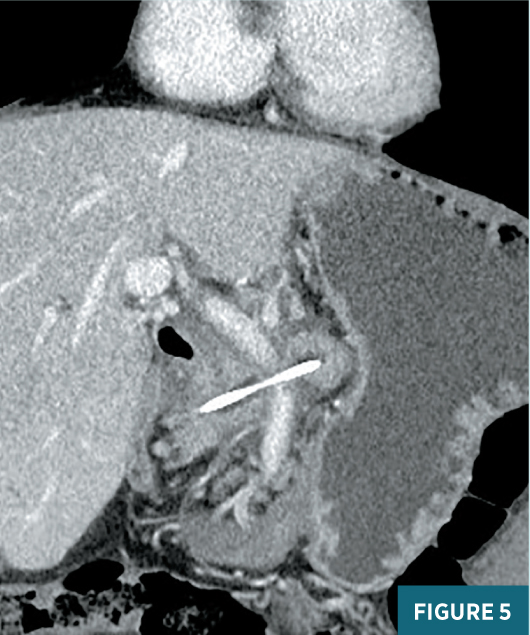
Chest radiograph obtained in the ED demonstrated a very large left pleural effusion (Figure 1). The amylase level in the tapped pleural fluid was >2,000 U/L. Subsequent CT of the abdomen demonstrated sequelae of chronic pancreatitis including pseudocysts near the pancreatic head, a dilated main pancreatic duct, and atrophic parenchyma (Figure 2). The coronal CT image shows a fistula at midline extending from the pancreatic region to the left pleural space (Figure 3). Endoscopic retrograde cholangiopancreatography (ERCP) was unsuccessful in placing a stent in the pancreatic duct, due to disruption in the duct. The patient was managed conservatively with medical therapy requiring a prolonged hospital stay. The left pleural effusion decreased significantly; however, the patient developed a new large right pleural effusion. Magnetic resonance cholangiopancreatography (MRCP) demonstrated a large pancreaticopleural fistula (PPF) and a right pleural effusion (Figure 4). Repeat ERCP was successful in placing a stent in the pancreatic duct with subsequent resolution of the fistula and pleural effusions. Follow-up coronal CT image showed the pancreatic duct stent and a significant decrease in pleural effusions (Figure 5).
Diagnosis
PPF.
Discussion
PPF is a rare complication of acute and chronic pancreatitis, more commonly of chronic pancreatitis. It occurs in only 0.4% of patients with pancreatitis. PPF is considered a type of internal fistula, wherein the pancreatic secretions drain directly into the pleural cavity. PPF develops from rupture of the pancreatic duct or a pseudocyst. Owing to enzymatic activity, exocrine pancreatic secretions can dissect through fascial planes and can enter the pleural space via the aortic/esophageal hiatus or through the diaphragmatic muscle itself.
The most common cause of chronic pancreatitis leading to PPF is alcohol abuse (around 80%). A smaller percentage of cases may be due to biliary stones, trauma, or idiopathic pancreatitis.
The common clinical presentation is that of recurrent large pleural effusions in the setting of acute or chronic pancreatitis. Patients with PPF usually present with respiratory symptoms such as dyspnea, more so than abdominal symptoms.
History and physical examination are nonspecific and further evaluation with imaging and laboratory work-up is required. The chest radiograph usually shows unilateral or sometimes bilateral pleural effusions. Left pleural effusion is more common, accounting for 76% of cases. Bilateral pleural effusions are less common and reported in only 14% of cases.
Pleural effusions tend to recur despite repeated thoracentesis. The level of pleural fluid amylase is generally above 1,000 U/L (normal levels <150 U/L), which may suggest PPF in the absence of malignant cells. When suspected, CT, ERCP, or MRCP can be performed for the visualization of the fistula. Although CT has a low sensitivity (approximately 47%) for detecting PPF, it is useful in demonstrating pancreatitis changes including parenchymal atrophy, calcifications, ductal dilatation, and pseudocysts. ERCP has a sensitivity of approximately 78% in detecting fistulas. It can provide information about the ampulla, in addition to depicting ductal anatomy. Moreover, it has the advantage of allowing endoscopic therapeutic interventions. However, ERCP is an invasive procedure with risk of complications such as infection, bleeding, pancreatitis, and perforation. It is also unable to clearly demonstrate a fistula, if the site of ductal disruption is distal to strictures.
MRCP has a sensitivity of approximately 80% in detecting fistulas, and it is the imaging modality of choice, being noninvasive and providing visualization of fistulas beyond strictures. Furthermore, changes of pancreatic parenchyma and surrounding tissue can also be shown and can help to stratify further management.
PPF are treated initially with conservative medical therapy, which includes octreotide, somatostatin, total parenteral nutrition, antibiotics, and thoracentesis. The next line of management is ERCP-guided stent placement in the pancreatic duct. Surgery is the last resort, if medical and endoscopic treatments fail. Pancreatic resection and enteropancreatic anastomosis constitute methods of surgical intervention to achieve drainage of pancreatic secretions.
— Pramod Gupta, MD, is a staff radiologist at the Dallas VA Medical Center.
— Robert Sims, MD, is a staff radiologist at the Dallas VA Medical Center.
Resources
1. Ali T, Srinivasan N, Le V, Chimpiri AR, Tierney WM. Pancreaticopleural fistula. Pancreas. 2009;38(1):e26-e31.
2. Aswani Y, Hira P. Pancreaticopleural fistula: a review. JOP. 2015;16(1): 90-94.
3. McCarthy S, Pellegrini CA, Moss AA, Way LW. Pleuropancreatic fistula: endoscopic retrograde cholangiopancreatography and computed tomography. AJR Am J Roentgenol. 1984;142(6):1151-1154.
4. Safadi BY, Marks JM. Pancreatic-pleural fistula: the role of ERCP in diagnosis and treatment. Gastrointest Endosc. 2000;51(2):213-215.
5. Vyas S, Gogoi D, Sinha SK, Singh P, Yadav TD, Khandelwal N. Pancreaticopleural fistula: an unusual complication of pancreatitis diagnosed with magnetic resonance cholangiopancreatography. JOP. 2009;10(6):671-673.
6. Dhebri AR, Ferran N. Nonsurgical management of pancreaticopleural fistula. JOP. 2005;6(2):152-161.
7. Roberts KJ, Sheridan M, Morris-Stiff G, Smith AM. Pancreaticopleural fistula: etiology, treatment and long-term follow-up. Hepatobiliary Pancreat Dis Int. 2012;11(2):215-219.

