 On the Case
On the Case
By Alex Merkulov, MD
Radiology Today
Vol. 20 No. 7 P. 30
History
A 30-year-old nulligravid and asymptomatic woman with a BRCA1 gene mutation was found to have a nonspecific liver mass on her screening breast MRI. She was on combination birth control which contained estrogen and progestin for oral contraception. The patient was referred for an MRI of the abdomen without and with IV contrast (5 mL Gadavist) to characterize the liver mass.
Findings
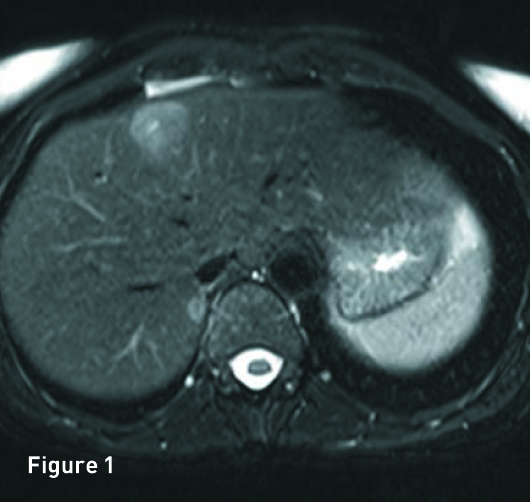
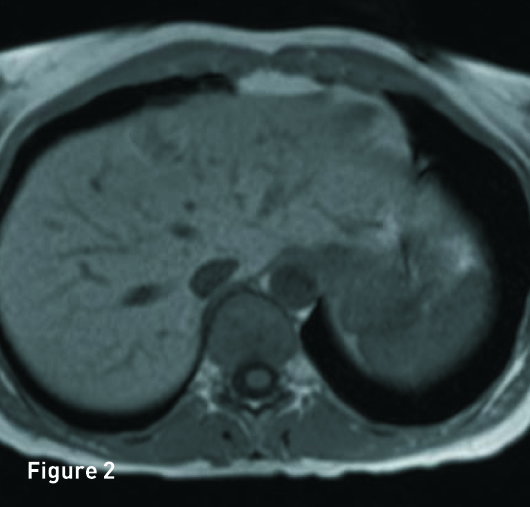
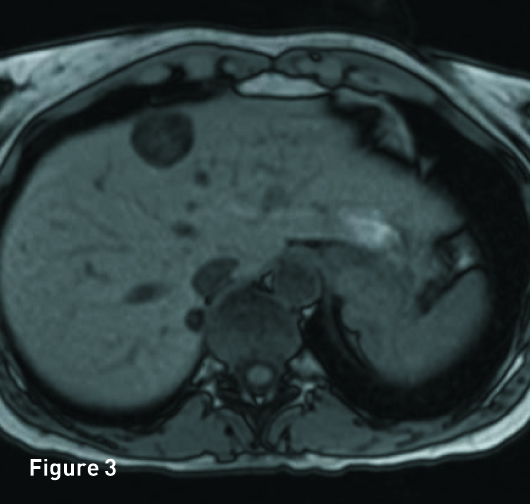
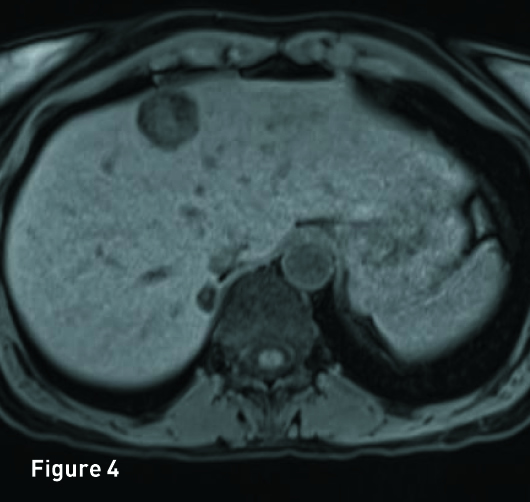
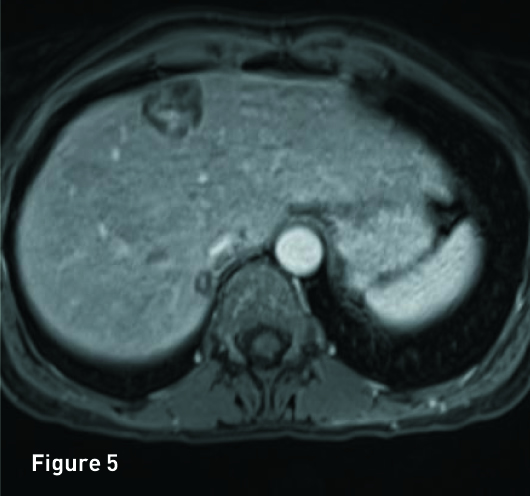
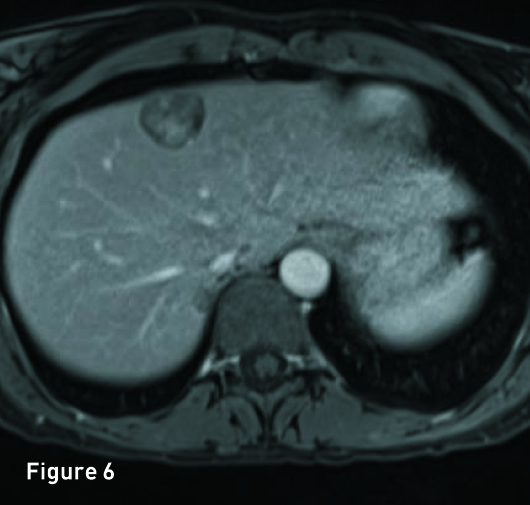
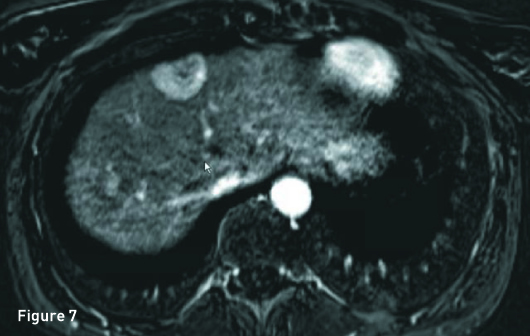
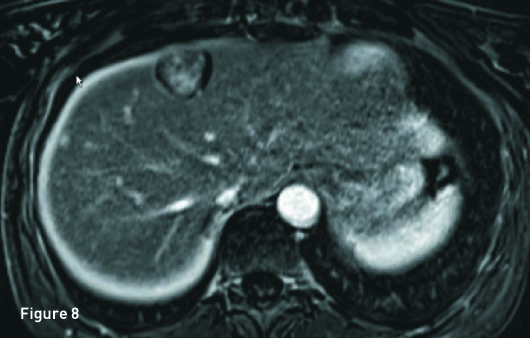
The liver is of normal volume with no cirrhosis or hepatic steatosis. In segment 4A there is a 2.9-cm T2 hyperintense (Figure 1), T1 in-phase heterogeneous slightly hyperintense mass (Figure 2) with significant signal drop out on out-of-phase imaging secondary to intralesion microscopic fat (Figure 3). The mass demonstrates heterogeneous avid arterial phase enhancement (Figures 4, pre-, and 5, arterial phase), which partially persists on the portal venous phase (Figure 6). Subtracted arterial phase and portal venous phase images are provided (Figures 7 and 8). A second, smaller mass, which is morphologically similar to the segment 4A mass, is located in segment 7 posteriorly to the inferior vena cava.
Diagnosis
Inflammatory hepatic adenoma (HA).
Discussion
HA is a rare benign tumor that is mainly found in women of child-bearing age. It is the second most frequent hepatocellular tumor in young women after focal nodular hyperplasia (FNH). HA is strongly related to current and recent—typically first-generation and high-dose—use of oral contraceptives (OC). The risk of developing HA increases with the duration of OC use, and tumor regression can occur after discontinuation of OC. Non-OC-related causes of HA include familial insulin-dependent diabetes, Fanconi anemia, glycogen storage diseases, and hormonal stimulation from other sources, eg, anabolic steroid use by body builders, gynecological tumors, or pregnancy.
HAs are usually solitary (70% to 80% of cases), subcapsular, often round, well-defined pseudo-encapsulated masses. Occasional dystrophic calcification may be present. When multiple, usually >10 adenomas, the term hepatic adenomatosis is used.
It may be difficult to distinguish HA from other benign or malignant liver tumors. Large adenomas have a marked tendency to hemorrhage and may undergo malignant transformation to hepatocellular carcinoma (HCC).
HA is generally asymptomatic. Laboratory tests for liver function are usually normal. Because of the different therapeutic options, HA must be distinguished from other hypervascular lesions (HCC, fibrolamellar HCC, FNH, and metastases) that may occur in young adults without cirrhosis.
HAs are currently categorized in four distinct genetic and pathologic subtypes as follows: inflammatory hepatocellular adenomas, hepatocyte-nuclear-factor-1-alpha-mutated hepatocellular adenomas, and beta-catenin-mutated hepatocellular adenomas. Finally, HAs without any genetic abnormalities are categorized in the unclassified subtype.
Inflammatory HA morphology and prognosis is different from other HA subtypes. It is the most common subtype of HA (40% to 50%), occurs most commonly in women with OC usage, and has the highest incidence of hemorrhage amongst HA subtypes.
In general, HAs appear circumscribed and isoattenuating to the liver on CT. After contrast administration, they demonstrate transient, relatively homogenous arterial-phase enhancement returning to near isodensity on portal venous and delayed imaging. Calcification may be seen in areas of old hemorrhage (5% to 10% of cases).
On MRI, HAs are variable on T1-weighted images and can range from hyper-, iso-, to hypointense. On T2, HAs are mildly hyperintense (47% to 74%) and can demonstrate signal drop out on out-of-phase imaging secondary to the presence of microscopic fat. On dynamic postcontrast imaging, HAs show early arterial enhancement and become nearly isointense to the liver on delayed images.
As a general rule, if a HA is <5 cm in size, discontinuation of OC and radiological follow-up is acceptable; if the lesion is >5 cm or near the hepatic surface, due to the recognized risk of rupture and hemorrhage, surgical resection is the treatment of choice. Pregnancy should be avoided due to increased risk of rupture.
— Alex Merkulov, MD, is an associate professor of radiology at UCONN Health.
Resources
1. Bioulac-Sage P, Balabaud C, Zucman-Rossi J. Subtype classification of hepatocellular adenoma. Dig Surg. 2010;27(1):39-45.
2. Brancatelli G, Federle MP, Vullierme MP, Lagalla R, Midiri M, Vilgrain V. CT and MR imaging evaluation of hepatic adenoma. J Comput Assist Tomogr. 2006;30(5):745-750.
3. Grazioli L, Morana G, Kirchin MA, Schneider G. Accurate differentiation of focal nodular hyperplasia from hepatic adenoma at gadobenate dimeglumine-enhanced MR imaging: prospective study. Radiology. 2005;236(1):166-177.
4. Paulson EK, McClellan JS, Washington K, Spritzer CE, Meyers WC, Baker ME. Hepatic adenoma: MR characteristics and correlation with pathologic findings. AJR Am J Roentgenol. 1994;163(1):113-116.

