 On the Case
On the Case
By Krithika Srikanthan, MD, and Alex Merkulov, MD
Radiology Today
Vol. 22 No. 5 P. 30
History
A 53-year-old female smoker was referred for diagnostic mammography and ultrasound imaging for a new indeterminate left breast subareolar mass identified on screening mammography. The patient had a personal history of left upper lobe adenocarcinoma status post lobectomy in 2018 and COPD. She continued to smoke one cigarette per day and also drank about one pint of alcohol daily. She reported using albuterol three times weekly and complained of a productive cough with tenacious sputum, occasional wheezing, and shortness of breath with exertion.
Findings
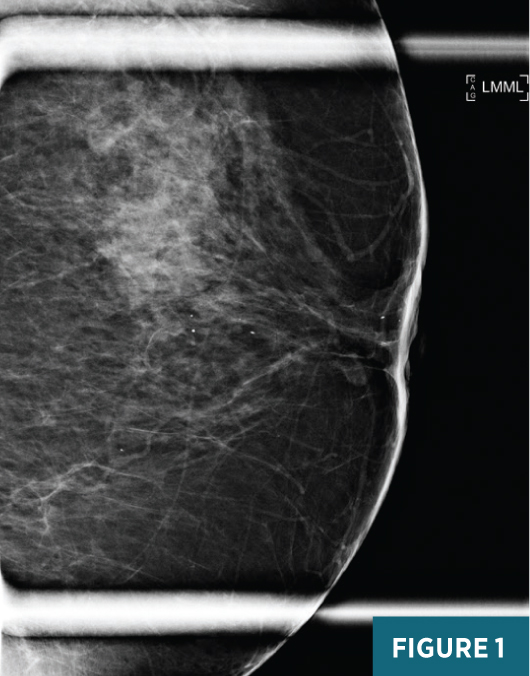
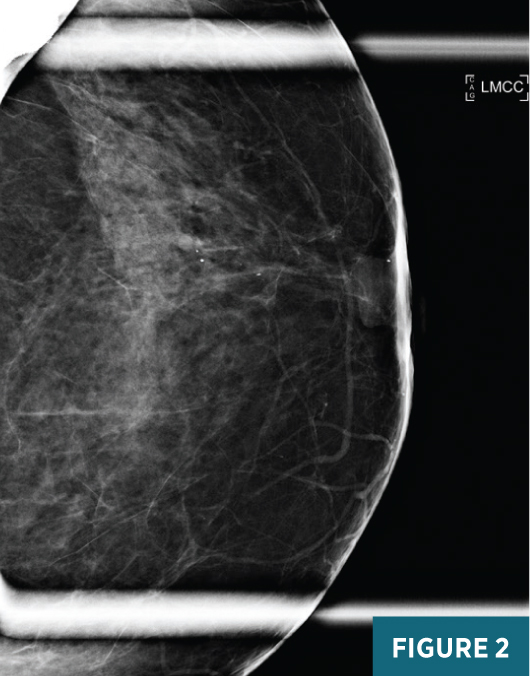
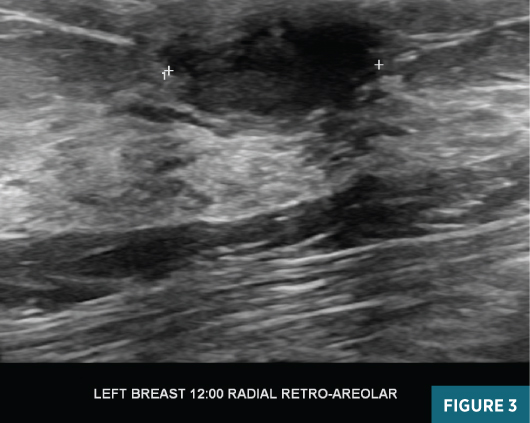
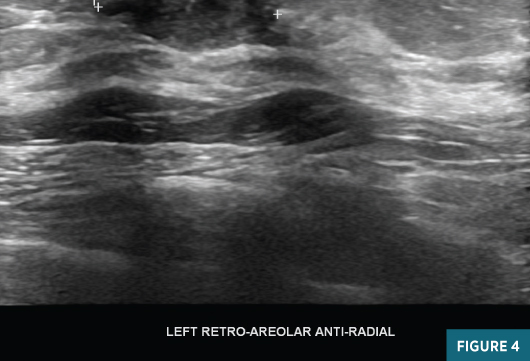
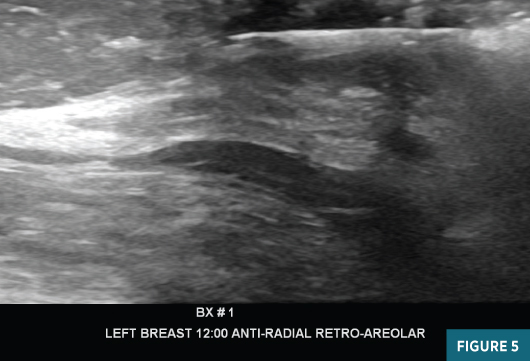
Left breast magnification craniocaudal and mediolateral views demonstrated an equal-density circumscribed 1.6-cm subareolar mass abutting the posterior aspect of the nipple. Several typically benign coarse calcifications were present in the subareolar breast and at anterior depth (Figures 1 and 2). Targeted grey scale imaging ultrasound revealed a subareolar 1.6-cm hypoechoic, nonvascular, parallel mass with angular margins (Figures 3 and 4). An ultrasound guided-biopsy was recommended and performed using a 14-gauge spring-loaded needle followed by biopsy marker clip placement (Figure 5).
Diagnosis
Squamous metaplasia of lactiferous ducts (SMOLD)/Zuska’s disease.
Discussion
SMOLD, also known as nonpuerperal subareolar mastitis and abscess or Zuska’s disease, is a rare, benign, chronic inflammatory process of the lactiferous ducts postulated to be due to lactiferous duct obstruction and resultant squamous metaplasia and hyperplasia.1 Although this is a benign entity, it is often associated with prolonged morbidity. SMOLD represents only 1% to 2% of all symptomatic breast processes; therefore, breast imagers may be unfamiliar with this disease and presentation. Diagnosis and appropriate medical surgical treatment is often delayed or prolonged.2
The pathogenesis of SMOLD involves squamous metaplasia of the cuboidal epithelium lining the lactiferous ducts. The squamous lining produces large amounts of keratin that obstructs and dilates the ducts, leading to acute inflammatory infiltrates and cellular debris. These ducts can then become infected as a result of stasis and bacterial invasion, which leads to abscess formation and fistula formation in up to one-third of patients. Abscess and persistent duct obstruction lead to continued accumulation of keratin debris and chronic inflammation. As the breast tissue heals, cytokines are secreted by inflammatory cells, inducing fibrosis.2
SMOLD can affect a wide age range, though peak incidence occurs in the fourth decade. While this can rarely present in males, 95% of patients are women.2 A strong correlation has been reported with cigarette smoking, and an association with inadequate vitamin supplementation (particularly vitamin A) has also been suggested.3,4 Unlike lactation breast abscesses, parity and lactation do not have a significant association, and SMOLD recurs more frequently (>50%), often requiring multiple drainage or surgical procedures.2 Higher rates of disease recurrence and subsequent complicated recovery have been linked to the presence of mixed flora, anaerobic bacteria, Proteus organisms, and cigarette smoking.5
Clinically, younger patients tend to present with breast pain, possibly due to acute periductal inflammation and/or palpable masses. When masses are present, they are frequently associated with erythema. Older patients present with less pain and palpable subareolar masses, possibly reflecting less acute inflammation and greater amounts of fibrosis. On physical exam, masses may be poorly defined and fixed to the adjacent tissue, due to fibrosis, and may have nipple retraction, raising suspicion for malignancy. Approximately 15% to 20% of patients report variable-colored and consistent nipple discharge.2
Possible mammographic findings include skin thickening, mass (irregular or circumscribed), focal or diffuse asymmetry, or normal mammogram. Lesions range in size from 1 to 5 cm. Ultrasound findings include complex cystic lesions (approximately 50% of cases) and nonspecific hypoechoic masses.6 Fistula tracts can sometimes be identified. Skin thickening is very commonly seen with ultrasound.5 In many instances, even when mammographic findings are normal, ultrasound may reveal small collections.2
The main differential diagnosis in a patient with skin thickening and a subareolar mass is malignancy, particularly inflammatory breast cancer. Key differences include differences in patient demographics. Patients with inflammatory breast cancers are approximately one decade older than SMOLD patients at time of diagnosis. On physical exam, inflammatory breast cancer classically presents with peau d’orange or erythema and mass in the upper outer quadrant, rather than retroareolar as in SMOLD. Mammographic findings with SMOLD and inflammatory breast cancer can be similar with skin thickening, prominent trabecula, edema, asymmetric density, or normal. On ultrasound, however, SMOLD presents with subareoalar solid and cystic masses or collections, whereas inflammatory breast cancer is much more likely to present with a solid mass on ultrasound (approximately 80% of the time).2
Contrast-enhanced breast MRI has also been shown to have high accuracy in defining the primary lesion and can help differentiate between SMOLD and inflammatory breast cancer, using kinetic curves and location of masses.7 Inflammatory breast cancer has been shown to have more initial early enhancement than SMOLD and more frequently shows washout kinetics, whereas SMOLD exhibits plateau or persistent kinetics. Regarding location, SMOLD more frequently is subareolar, and inflammatory breast cancer is more often found in posterior depth of the breast.8
Given how uncommon SMOLD is and lack of specificity of the imaging findings, histologic diagnosis is key to the diagnosis and treatment of SMOLD. Treatment is debated, though a growing body of literature supports resection of the obstructed subareolar duct(s) and associated abscess and fistula, due to reported lower recurrence rates. Also, given the strong association with smoking, smoking cessation is recommended to prevent future incidents of SMOLD involving the remaining lactiferous ducts.2
— Krithika Srikanthan, MD, is a radiology resident at UConn Health at the University of Connecticut.
— Alex Merkulov, MD, is an associate professor of radiology at UConn Health at the University of Connecticut.
References
1. Gimenez Sanders, MA. Breast inflammatory SMOLD. Pathology Outlines website. https://www.pathologyoutlines.com/topic/breastsmold.html. Updated May 6, 2021.
2. Kasales CJ, Han B, Smith JS Jr, Chetlen AL, Kaneda HJ, Shereef S. Nonpuerperal mastitis and subareolar abscess of the breast. AJR Am J Roentgenol. 2014;202(2):W133-W139.
3. Schäfer P, Fürrer C, Mermillod B. An association of cigarette smoking with recurrent subareolar breast abscess. Int J Epidemiol. 1988;17(4):810-813.
4. Meguid MM, Oler A, Numann PJ, Khan S. Pathogenesis-based treatment of recurring subareolar breast abscesses. Surgery. 1995;118(4):775-782.
5. Serrano LF, Rojas-Rojas MM, Machado FA. Zuska's breast disease: breast imaging findings and histopathologic overview. Indian J Radiol Imaging. 2020;30(3):327-333.
6. Lequin MH, van Spengler J, van Pel R, van Eijck C, van Overhagen H. Mammographic and sonographic spectrum of non-puerperal mastitis. Eur J Radiol. 1995;21(2):138-142.
7. Le-Petross HT, Cristofanilli M, Carkaci S, et al. MRI features of inflammatory breast cancer. AJR Am J Roentgenol. 2011;197(4):W769-W776.
8. Renz DM, Baltzer PA, Böttcher J, et al. Magnetic resonance imaging of inflammatory breast carcinoma and acute mastitis. A comparative study. Eur Radiol. 2008;18(11):2370-2380.

