 On the Case
On the Case
By Paola Tabaro, MD; Prachi Patel, MBBS; and Alex Merkulov, MD
Radiology Today
Vol. 23 No. 7 P. 46
History
A 31-year-old woman presented with a palpable left upper outer breast mass. After a physical exam, she was referred for diagnostic left breast mammography and a targeted breast ultrasound. On ultrasound, an irregular 1:00/2:00 hypoechoic mass correlated as palpable, and the patient underwent an ultrasound guided percutaneous biopsy with histologic and immunohistochemical findings consistent with Rosai-Dorfman disease (RDD). Three years later, the patient presented with worsening left breast and left arm pain. She was referred to breast and orthopedic surgical specialists for an evaluation. She subsequently underwent diagnostic
mammography and ultrasound imaging of the left breast and musculoskeletal imaging with radiography and MRI.
Findings
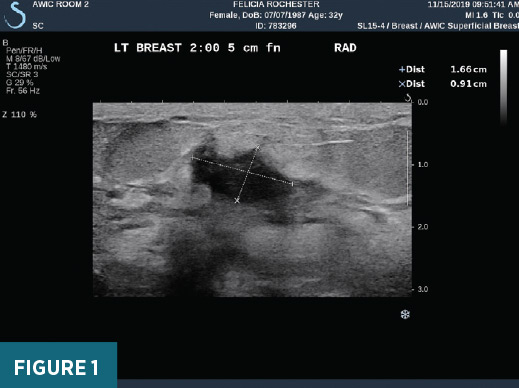
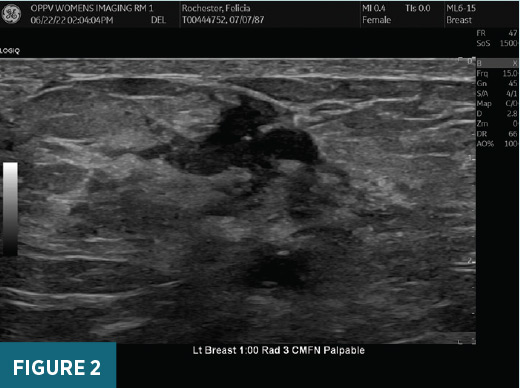
Left breast ultrasound imaging in 2019 demonstrated a 1.7 cm 1:00/2:00 slightly irregular hypoechoic mass in the area of clinical concern (Figure 1); this mass was biopsied with RDD on pathology. Left breast ultrasound imaging in 2022 demonstrated a 1.8 cm 1:00/2:00 hypoechoic
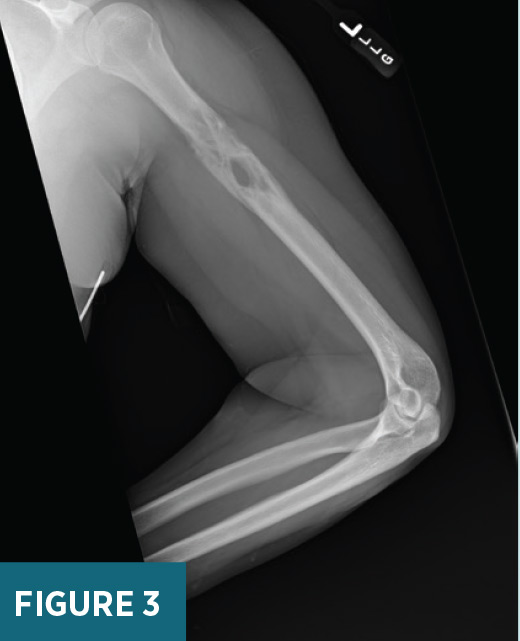
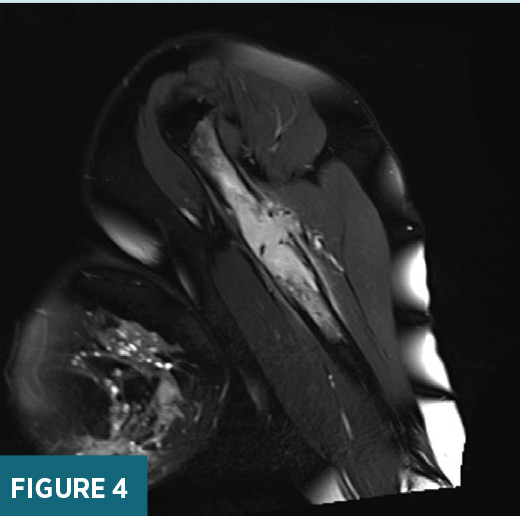
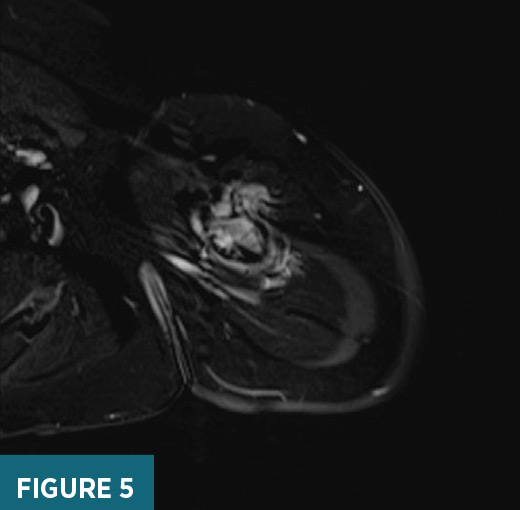
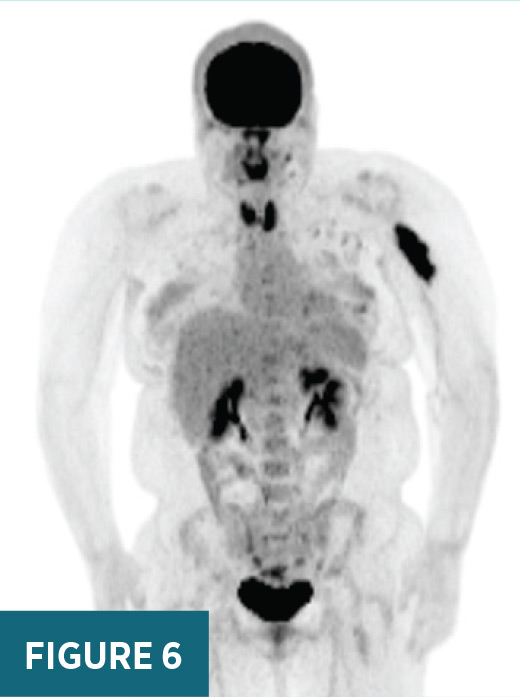
previously biopsied mass in the area of clinical concern (Figure 2). X-ray of the left humerus demonstrated a destructive lesion in the left diaphyseal humerus (Figure 3). MRI of the left arm demonstrated a T2 heterogeneously hyperintense, T1 hypointense enhancing 8.1 cm in long axis lesion within the proximal left diaphyseal humerus with bony expansion, internal septations, and cortical destruction (Figures 4 and 5). On subsequent PET/CT, an intensely FDG avid (maximum SUV 19.6) lytic lesion in the left diaphyseal humerus correlated with the mass on MRI imaging (Figure 6).
The patient underwent an open biopsy of the left humerus with pathology revealing histologic and immunohistochemical findings consistent with involvement of bone by patient’s previously diagnosed RDD of the left breast.
Diagnosis
Rosai-Dorfman disease
Discussion
RDD, also known as sinus histiocytosis, is an extremely rare benign disorder characterized by abnormal proliferation and accumulation of histiocytes in lymph nodes. RDD is characterized by painless lymphadenopathy and histiocytic proliferation.1 A specific disease etiology remains unclear, although prior case reports suggest that RDD may be incited by an abnormal immune response to viral agents, such as Epstein-Barr virus and herpes viruses, which may result in an abnormal proliferation of sinusoidal histiocytes.1 RDD has an estimated prevalence of 1/200,000 and is predominantly seen in children and young adults with an estimated mean age of 20 years at the time of diagnosis.2 Prior case reports have shown a predilection for the male gender and African ancestry.1
The most common clinical presentation of RDD is painless cervical lymphadenopathy. Less commonly, cases with extranodal involvement have been reported involving multiple organ systems, including skin, bones, central nervous system, kidneys, gastrointestinal tract, respiratory tract, cardiac system, and breast.1 The typical presentation of isolated RDD in the pediatric population mainly involves head and neck lymphadenopathy, which follows a benign self-limiting course. Patients who present at an older age and have underlying immunologic abnormalities are at increased risk for extranodal disease and a chronic relapsing disease course.3 The paranasal sinuses are the most common extranodal site, which mainly manifests as polypoid mucosal masses.1 Skin is another common extranodal site, with variable presentations in the form of erythematous lesions and/or dermal nodules.1 Intracranial disease can have variable manifestations and can sometimes mimic dural-based masses.1 Manifestations of RDD in the thorax and abdomen are less common but may appear similar to other multiorgan diseases such as lymphoma with evidence of multiple solid masses and lymphadenopathy on imaging leading to mass effect and eventual multiorgan failure.3 Osseous manifestations are rare, and patients with bone involvement tend to present with arthralgias.3 Patients with involvement of the lower respiratory tract, hepatic system, or renal system have a higher mortality rate, which can be as high as 40%.3 Breast involvement is extremely rare and typically presents as a painless palpable breast mass. A history of preceding systemic symptoms may be present, such as fever, weight changes, and/or axillary lymphadenopathy.4 It is important to note that there are also cases of isolated RDD without lymph node involvement.
Diagnosis of RDD entails a combination of clinical presentation, imaging features, and biopsy. The specific pathologic features of RDD show sinus expansion of large histiocytes with a pale cytoplasm, a large hypochromatic nucleus, and a prominent nucleolus. Pathological evidence of emperipolesis (nondestructive phagocytosis of lymphocytes and erythrocytes) has also been established, although is not considered pathognomonic for this disease.2 Immunohistochemical staining of RDD histiocytes is positive for CD68, CD14, HLA-DR, fascin, CD163, and S100 and is typically negative for CD1a, which aid in distinguishing RDD from different histiocytotic disorders. Positive histology for RDD is required but not sufficient to make the final diagnosis, which also depends on the clinical presentation, findings on imaging, and the exclusion of primary malignant disorders that may appear identical to RDD.2
Imaging findings vary according to the systemic manifestations. Our patient demonstrated extranodal involvement in the breast and proximal humerus. Osseous manifestations on radiographs typically reveal a lytic intramedullary lesion which may be associated with surrounding sclerosis.1 Breast involvement presents as a morphologically suspicious mass on ultrasound.5 In the majority of reported breast cases, targeted ultrasound showed a hypoechoic mass with irregular borders, which meets the criteria for biopsy. Mammographic findings can be negative or variable, with some studies reporting high-density spiculated masses or focal asymmetries.5,6 FDG-avid homogeneous soft tissue masses or osseous lesions are seen on PET/CT.7
When establishing a diagnosis of RDD, it is essential to exclude underlying conditions that may appear clinically and radiologically similar. The main differential diagnoses that should be excluded include hemophagocytic syndromes, malignant histiocytosis, and lymphoma. RDD is a heterogenous entity with a wide range of clinical phenotypes that can occur in isolation or in association with other autoimmune entities, with treatment recommendations varying according to the specific clinical presentation. Groups of clinical experts, which include pathologists, hematologists, oncologists, and internists, have tried to establish a consensus on different treatment recommendations. A direct uniform approach has not been established.2 Upon review of the literature, follow-up is recommended for patients with uncomplicated adenopathy, asymptomatic cutaneous RDD, and as postoperative management for resected local disease.2 Surgical excision of lesions is usually not necessary, although it is commonly performed given the limited data available. It is also important to recall that patients with localized RDD of the breast have shown good outcomes without any surgical intervention. Optional treatment with corticosteroids, chemotherapy, and immunomodulators have been recommended for patients with symptomatic RDD or disseminated and recurring cases.
— Paola Tabaro, MD, is a radiology resident at UConn Health at the University of Connecticut.
— Prachi Patel, MBBS, is a research assistant from Maharaja Sayajirao University of Baroda in India.
— Alex Merkulov, MD, is an associate professor of radiology at UConn Health.
References
1. Vaidya T, Mahajan A, Rane S. Multimodality imaging manifestations of Rosai-Dorfman disease. Acta Radiol Open. 2020;9(8):2058460120946719.
2. Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890.
3. Mar WA, Yu JH, Knuttinen MG, et al. Rosai-Dorfman disease: manifestations outside of the head and neck. AJR Am J Roentgenol. 2017;208(4):721-732.
4. Green I, Dorfman RF, Rosai J. Breast involvement by extranodal Rosai-Dorfman disease: report of seven cases. Am J Surg Pathol. 1997;21(6):664-668.
5. Kuzmiak CM, Koomen M, Lininger R, Pisano E. Rosai-Dorfman disease presenting as a suspicious breast mass. AJR Am J Roentgenol. 2003;180:(6):1740-1742.
6. Iancu G, Gica N, Mustata LM, Panaitescu AM, Vasile D, Peltecu G. Rosai-Dorfman disease: breast involvement—case report and literature review. Medicina (Kaunas). 2021;57(11):1167.
7. Yu JQ, Zhuang H, Xiu Y, Talati E, Alavi A. Demonstration of increased FDG activity in Rosai-Dorfman disease on positron emission tomography. Clin Nucl Med. 2004;29(3):209-210.
