 On the Case
On the Case
By Racquel Helsing, MD; Kevin Haines, DO; and Alex Merkulov, MD
Radiology Today
Vol. 26 No. 6 P. 32
History
A 35-year-old woman with a long-standing history of systemic lupus erythematosus (SLE) was initially diagnosed at age 18 following the development of a malar rash. Her early management included hydroxychloroquine (Plaquenil) and prednisone, and she was later found to have lupus nephritis, at which point mycophenolate mofetil (Cellcept) was added. Her serologic profile has consistently shown positive antinuclear antibody (ANA) (1:1280 speckled), anti-Ro, anti-La, anti-Smith, antiribonucleoprotein, and antidouble-stranded (ds)DNA antibodies, along with low complement levels, positive lupus anticoagulant, and low-titer anticardiolipin antibodies. She discontinued her medications in 2021 due to insurance barriers and remained off treatment into 2023. In May 2024, she presented to the emergency department with abdominal pain, nausea, vomiting, and diarrhea. She was admitted for observation and discharged the following morning after receiving supportive care overnight. However, she returned to the emergency department the next day with an inability to tolerate oral intake, chills, and worsening abdominal pain. On evaluation, she was febrile, tachycardic, and exhibited abdominal distension with lactic acidosis. CT of the abdomen and pelvis was ordered.
Findings
Contrast-enhanced CT of the abdomen and pelvis demonstrated prominent circumferential mural thickening involving the gastric antrum, duodenum, and the entire length of the small bowel, accompanied by adjacent mesenteric fluid (Figures 1-3). Additional mural thickening was noted in the esophagus (Figure 4) and cecum. These findings, in the context of known SLE, were highly suggestive of small bowel vasculitis.
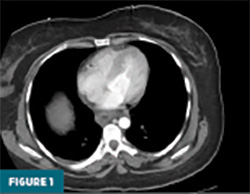
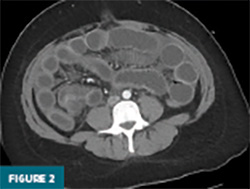
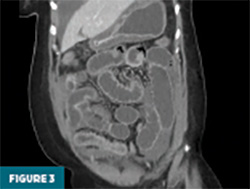
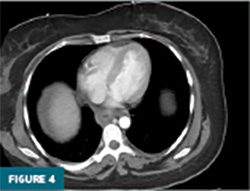
The remaining colon appeared collapsed, consistent with the patient’s history of diarrhea. A small-to-moderate volume of free intraperitoneal fluid was present, with tracking into the extraperitoneal pelvic spaces (Figure 5). A trace right pleural effusion was noted, along with mild bilateral hydronephrosis and proximal ureterectasis (Figure 6).
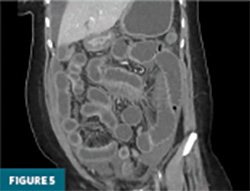
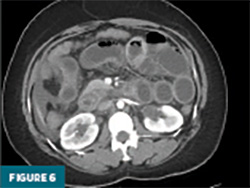
Taken together, the constellation of findings, including diffuse small bowel and gastric mural thickening, esophagitis, ascites, and mild upper urinary tract abnormalities, in a patient with established SLE, strongly indicated a multisystem lupus flare involving the gastrointestinal and possibly genitourinary tracts. The presence of segmental small bowel wall thickening with submucosal edema, mural hyperenhancement, mesenteric vascular engorgement, and ascites was consistent with lupus enteritis.
She was admitted to the surgical service for further evaluation. A nasogastric tube was placed to low wall suction, and she was started on intravenous antibiotics. Due to persistent tachycardia without clinical improvement, she was taken to the operating room for diagnostic laparoscopy. Laparoscopic findings demonstrated diffuse small bowel dilation without evidence of necrosis, narrowing, obstruction, or closed-loop pathology. She was admitted to the ICU postoperatively and intubated.
Rheumatology and gastroenterology were consulted. Rheumatologic workup revealed positive ANA, dsDNA, anticardiolipin, and lupus anticoagulant antibodies, low C3 and C4 levels, and elevated erythrocyte sedimentation rate, consistent with an active lupus flare. Blood, urine, and stool cultures were negative. High-dose intravenous corticosteroids were initiated with subsequent clinical improvement. The patient was successfully extubated, and she continued to improve under medical management. Endoscopic evaluation was deferred, given her favorable response to steroids.
Diagnosis
Lupus Enteritis
Discussion
Lupus enteritis, a rare yet serious complication of SLE, involves inflammation of the intestinal wall that can manifest as abdominal pain, nausea, vomiting, diarrhea, or, more alarmingly, bowel ischemia and perforation. Although it most commonly affects the small intestine, large bowel involvement has been described, particularly in older patients. The underlying pathophysiology is attributed to immune complex deposition and complement- mediated vasculitis of small- and medium-sized mesenteric vessels, leading to bowel wall ischemia and edema.
SLE is a multisystem autoimmune disorder characterized by a wide spectrum of clinical manifestations, with gastrointestinal involvement seen in up to 40% of patients. While esophageal dysmotility and dysphagia are common, lupus enteritis— also referred to as mesenteric vasculitis— represents a far less frequent but potentially life-threatening entity. Two phenotypes have been proposed: a smallintestine– dominant form that may result in mesenteric thrombosis, infarction, or perforation, and a large-intestine–dominant form, which may mimic pseudoobstruction due to chronic ischemia and impaired motility. An additional, albeit rare, presentation includes peritonitis with acute ascites during a flare—an ominous finding that warrants exclusion of ischemia or perforation.
The clinical presentation of lupus enteritis often mimics more common abdominal pathologies such as infectious enteritis, mesenteric ischemia, or bowel obstruction. This overlap underscores the importance of maintaining a high index of suspicion in SLE patients with acute gastrointestinal complaints, even in the absence of other systemic disease markers. Notably, lupus enteritis may precede or serve as an isolated manifestation of disease flare.
Diagnostic imaging, particularly contrast-enhanced CT, is central to establishing the diagnosis. Classic CT findings include circumferential bowel wall thickening, the “target sign” or “double halo sign,” mesenteric edema, and sometimes ascites. These features were evident in our patient and played a crucial role in guiding the diagnosis. While imaging can be strongly suggestive, the diagnosis ultimately relies on a combination of imaging, clinical context, and response to immunosuppressive therapy.
Management revolves around prompt immunosuppression. High-dose intravenous corticosteroids are the first-line treatment, often yielding rapid symptomatic and radiologic improvement. In steroidrefractory cases or patients with recurrent disease, additional immunosuppressive agents such as cyclophosphamide or azathioprine may be required. Our patient responded well to corticosteroid therapy, and maintenance immunosuppression was instituted to mitigate recurrence risk.
Importantly, lupus enteritis carries a significant risk of recurrence, with some series citing relapse rates as high as 25%. As such, longitudinal management of underlying SLE and regular monitoring are essential. The potential for severe complications, including bowel infarction and perforation, further supports the case for ongoing immunologic control and close follow-up.
This case also underscores the importance of interdisciplinary collaboration involving rheumatology, gastroenterology, radiology, and surgery. Such coordination ensures accurate diagnosis, appropriate treatment, and surveillance. Furthermore, in ambiguous or nonresolving cases, surgical exploration may be necessary, though it is typically reserved for those who fail medical management or in whom perforation is suspected.
In summary, lupus enteritis is a serious yet treatable gastrointestinal manifestation of SLE. Prompt recognition, accurate imaging interpretation, and swift initiation of corticosteroid therapy are critical to reducing morbidity. Clinicians should remain vigilant for this diagnosis in SLE patients with acute abdominal pain, as early intervention can substantially alter the clinical course. Further research is needed to refine diagnostic criteria, predict recurrence, and establish standardized therapeutic pathways.
Racquel Helsing, MD, is a radiology resident at UConn Health at the University of Connecticut in Farmington.
Kevin Haines, DO, is a radiology resident at UConn Health.
Alex Merkulov, MD, is an associate professor of radiology at UConn Health.
Resources
1. Kaneko Y, Hirakata M, Suwa A. et al. Systemic lupus erythematosus associated with recurrent lupus enteritis and peritonitis. Clin Rheumatol. 2004;23(4):351–354.
2. Jotwani PM, Patel AV. Radiographic findings of lupus related entero-colitis. Clin Rheumatol. 2022;41(5):1617-1618.
3. Tian XP, Zhang X. Gastrointestinal involvement in systemic lupus erythematosus: insight into pathogenesis, diagnosis and treatment. World J Gastroenterol. 2010;16(24):2971-2977.

