.jpg)
On the Case
By Jamil Ahmed, MD; Nikhil Kanthala, DO; and Laura Gregorio, MD
Radiology Today
Vol. 25 No. 1 P. 32
History
Patient is a 44-year-old male with recent admission for symptoms related to anemia, abdominal pain, and melanotic stools. He was managed conservatively with a blood transfusion and followed with outpatient endoscopy, which revealed a large, ulcerated lesion in the stomach for which he was subsequently referred to advanced gastroenterology.
Findings
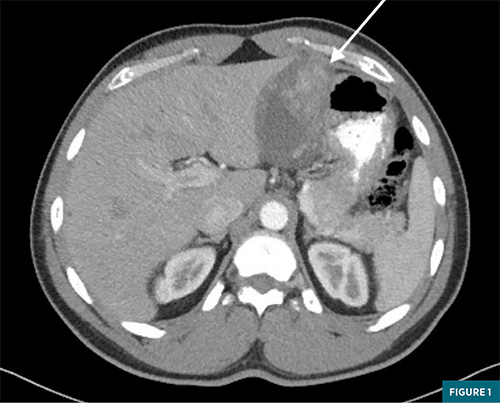
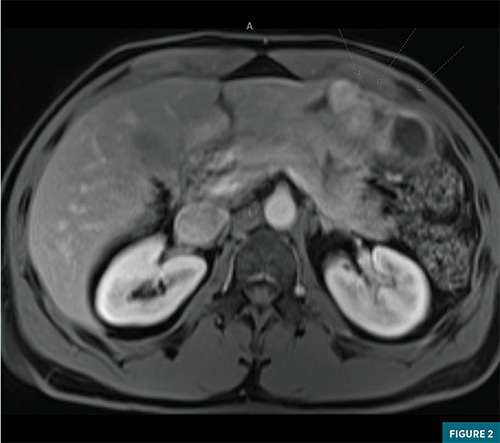
Advanced gastroenterology performed an endoscopy/endoscopic ultrasound, again finding a similar intramural ulcerated stomach mass, which measured 3.5 x 1.7 cm along the lesser curvature. Fine-needle aspiration was performed with pathology demonstrating clusters of neoplasm containing small vascular spaces and prominent perivascular epithelioid to spindle cells. These were positive for CD34, ERG, and smooth muscle actin. These cells were negative for Ki-67, desmin, CD138, CD68, HMB-45, SOX- 10, S100, MART 1, CD-117, cytokeratin, MOC-31, DOG1, beta catenin, and ALK. Per pathology, this mass was most consistent with a gastric glomus tumor. Both CT and MRI imaging with IV contrast were obtained of the abdomen with dedicated axial images provided in Figure 1 and Figure 2, respectively. CT showed a large heterogeneously enhancing intramural mass measuring 8.7 x 4.9 x 8.8 cm, arising from the lesser curvature of the stomach with intraluminal gastric extension. The mass did not appear to involve the gastroesophageal junction, and distally, it was fairly proximal to the pylorus. No evidence of metastatic disease was noted. MRI imaging showed a 9.1 x 5.4 x 8.8 cm mass along the lesser curvature with T2 hyperintensity, restricted diffusion, and a heterogeneous enhancement. The patient met with a surgical oncology specialist to discuss resection options but ultimately elected not to undergo surgery.
Left lower extremity skin biopsy results demonstrated a predominance of perivascular IgA over C3 deposition within the spectrum of an IgA-mediated small-vessel vasculitis (including Henoch-Schonlein purpura). Periodic acid-Schiff and Gram stains were negative for fungal elements or bacteria. Direct immunofluorescence studies revealed superficial perivascular IgA (2-3+, granular) and C3 (trace-1+, granular) with interstitial fibrinogen (3+) deposition; they were negative for definitive IgG or IgM deposition.
Prior autoimmune workup demonstrated normal C3, C4, negative antinuclear antibody, negative extractable nuclear antigen, negative double stranded- DNA, negative rheumatoid factor, negative cryo, negative myeloperoxidase/ proteinase-3/antineutrophil cytoplasmic antibodies, negative antiphospholipid antibodies, normal IgG4, normal IgA level, normal serum protein electrophoresis. Hepatitis B surface antigen, hepatitis B core antibody IgM, and hepatitis C antibody were also negative.
Upon admission, the patient was treated with a three-day pulse of IV solumedrol for IgA vasculitis with improvement in abdominal pain. In addition to clinical improvement, his inflammatory markers also gradually responded favorably. He was discharged on 40 mg of daily prednisone.
Diagnosis
Gastric glomus tumor
Discussion
Glomus tumors are rare mesenchymal tumors, postulated to arise from neuromyoarterial smooth muscle cells involved in thermal temperature regulation.1 They can occur in a variety of locations throughout the body, often in the distal extremities involving the nail bed, though they may also be seen in the middle ear, the reproductive organs, visceral abdominal organs, as well as the gastrointestinal system, namely the stomach.1-4 When considered in the gastrointestinal tract, they represent up to 1% of mesenchymal gastric tumors and are typically benign, though malignant cases have been reported.3 They do not demonstrate specific clinical, radiographic, or endoscopic features and may appear radiologically similar to the more commonly seen gastrointestinal stromal tumor (GIST), especially given their submucosal basis.3,4 Similar to a GIST, they may demonstrate an exophytic growth pattern along the gastric wall with irregular margins, central necrosis/ hemorrhage, and a heterogeneous enhancement pattern.5 As a result, the diagnosis typically relies on histopathological analysis either after initial biopsy or surgical resection.4 Other lesions that must be considered in the differential diagnosis include leiomyoma, schwannoma, neuroendocrine tumors, and lymphoma.5 Aside from cross-sectional imaging findings, as described above, endoscopic ultrasound may also be used in the initial workup of submucosal masses prior to surgical resection. One advantage stems from the ability to detect the layer of origin within the gastric wall, though this does not necessarily provide clarity in the diagnosis.6 If possible, biopsy might be performed in order to obtain histopathological samples prior to definitive treatment as in the case report of Tsagkataki et al, as well as ours, though, due to the intramural location, biopsy may not be possible.4,6 A variety of surgical approaches may be taken to remove the tumor, often based on the size, location, and suspicion of malignancy. These include mainly subtotal gastrectomy or wedge resection, using either open or laparoscopic techniques. Another approach that has been reported in the literature includes a laparoscopic endoscopy cooperative surgery, finding success in the treatment of glomus tumors, especially at the pylorus or GE junction. 3,7 Enucleation via endoscopy is also an option when the mass is located far from critical structures such as the porta hepatis, pylorus, and the lesser curvature of the stomach.8 The vast majority of these tumors tend to be benign, though there have been reported malignant cases.3 Factors demonstrated to be more predictive of malignancy include those with a deep location, a size greater than 2 cm, and mitotic atypia.9 Even when benign, glomus tumors may have a recurrence rate of up to 10% and thus require crosssectional imaging surveillance. When malignant, local recurrence and metastasis have been reported in up to 40% of cases, obviating the need for close clinical follow-up.3,10 Overall, this case highlights an important differential consideration when presented with a gastric mass that may appear similar to the more commonly encountered GIST. As discussed, while the majority are benign, malignancy has been reported, and therefore clinical and radiographic follow-up is critical to ensure lack of recurrence or metastasis.
— Jamil Ahmed, MD, is a vascular IR resident at Christiana Care in Delaware.
— Nikhil Kanthala, DO, is a diagnostic radiology resident at Christiana Care.
— Laura Gregorio, MD, is an attending radiologist at Christiana Care.
References
1. Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-188.
2. Tsagkataki ES, Flamourakis ME, Gkionis IG, et al. Gastric glomus tumor: a case report and review of the literature. J Med Case Rep. 2021;15(1):415.
3. Alsahwan AG, Alfaraj ZM, AlSafwani J, et al. Rare gastric neoplasm: malignant glomus tumor of the stomach. A case report. Int J Surg Case Rep. 2021;81:105802.
4. Papadelis A, Brooks CJ, Albaran RG. Gastric glomus tumor. J Surg Case Rep. 2016;2016(11):rjw183.
5. Gong J, Kang W, Zhu J, Xu J. CT and MR imaging of gastrointestinal stromal tumor of stomach: a pictorial review. Quant Imaging Med Surg. 2012;2(4):274-279.
6. Kang G, Park HJ, Kim JY, et al. Glomus tumor of the stomach: a clinicopathologic analysis of 10 cases and review of the literature. Gut Liver. 2012;6(1):52-57.
7. Aoba T, Kato T, Hiramatsu K, et al. A case of gastric glomus tumor resection using laparoscopy endoscopy cooperative surgery (LECS). Int J Surg Case Rep. 2018;42:204-207.
8. Chen KB, Chen L. Glomus tumor in the stomach: a case report and review of the literature. Oncol Lett. 2014;7(6):1790-1792.
9. Folpe AL, Fanburg–Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25(1):1-12.
10. Lin J, Shen J, Yue H, Li Q, Cheng Y, Zhou M. Gastric glomus tumor: a clinicopathologic and immunohistochemical study of 21 cases. Biomed Res Int. 2020;2020:5637893.

