.jpg)
Billing and Coding: 2024 Is Here … What You Need to Know
By Melody W. Mulaik, MSHS, CRA, RCC, RCC-IR, CPC, COC, FAHRA
Radiology Today
Vol. 25 No. 1 P. 6
The number of new codes introduced each year for radiology has continued to decrease. That is not necessarily a bad thing. It does provide opportunities for organizations to focus on other updates and issues of concern that may impact reimbursement and compliance. Read on for a high-level overview, not necessarily in priority order, of some key things to have on your radar as the new year begins.
Appropriate Use Criteria
The Protecting Access to Medicare Act of 2014 required CMS to establish a program to promote the utilization of appropriate use criteria (AUC) for advanced diagnostic imaging services. Advanced imaging services include diagnostic CT, MR, and nuclear medicine exams, including PET. AUC are designed to help clinicians select the most appropriate imaging study for a patient with a particular diagnosis or presenting symptom.
The program was originally scheduled to go into effect on January 1, 2017. The requirement was that ordering professionals consult AUC and communicate the results of the AUC consultation to the imaging provider (“furnishing professional”). After several delays, the program was officially implemented on January 1, 2020, with an educational and testing period where facilities and physicians could begin operational testing of claim submission with no risk of penalties for noncompliance. On July 7, 2022, CMS announced an indefinite delay to the AUC program penalty period. The educational and operations testing period continued in spite of the delay.
In the 2024 MPFS Final Rule, CMS officially paused the AUC program for reevaluation, ending the current educational and operations testing period. It did not indicate a time frame of when implementation efforts may resume, if at all. So, effective January 1, 2024, organizations should cease testing and no longer submit G codes or AUC modifiers.
Evaluation and Management Coding
CMS does not always agree or follow the American Medical Association (AMA) guidelines for evaluation and management (E/M) services. For example, CMS has been proposing to implement a definition of “substantitive portion” as more than half of the total provider time vs following the AMA definition of either time or medical decision making (MDM) to determine whether a service would be billed under the physician or the other qualified health professional (nurse practitioner or physician assistant). In an interesting turn of events, CMS has decided to forego its previously proposed and finalized definitions and align with the AMA’s CPT E/M guidelines for 2024.
CMS does acknowledge there can be instances where MDM is not easily attributed to a single physician or nonphysician practitioner when the work is shared; it does expect that whoever performs the MDM and subsequently bills the visit would appropriately document the MDM in the medical record to support billing the visit.
After delaying the implementation of its definition of “substantitive portion” several times, to avoid the administrative burden of time and resources spent preparing for potential policy changes that are delayed year after year, CMS is finalizing a revised definition of “substantive portion” of a split (or shared) visit to reflect the revisions to the CPT E/M guidelines. For Medicare billing purposes, the “substantive portion” means more than half of the total time spent by the physician and nonphysician practitioner performing the split (or shared) visit, or a substantive part of the MDM, except critical care visits, which only use time.
Payer Restrictions
Aetna recently published that effective December 1, 2023, “We won’t allow additional payment for E/M services billed by radiology providers.”1 Its publication goes on to list 16 separate radiology taxonomy codes and definitions. Its list includes all diagnostic radiology designations.
A taxonomy code is a unique 10-character code that designates a provider’s classification and specialization. This code is utilized when applying for a National Provider Identifier, commonly referred to as an NPI.
Interventional radiologists frequently and appropriately perform and bill for E/M visits. There are many interventional radiologists who are officially listed with payers with a diagnostic radiology taxonomy, which may put their reimbursement in jeopardy.
All interventionalists should be classified as 2085R0204X—vascular and interventional radiology. If you have a provider that is incorrectly classified, you can make changes on the CMS website.2
Discarded Drugs (JW/JZ Modifiers)
In certain circumstances, Medicare will pay for leftover drugs, including contrast materials and radiopharmaceuticals, which must be discarded or “wasted.” Medicare’s discarded drug policy is located in Chapter 17 of the Medicare Claims Processing Manual. It states that when a provider administers part of a single-use vial or other single- use package to a Medicare patient, and the rest of the container must be discarded, Medicare will pay both for the amount that was administered and the amount that was discarded. Note that this policy applies only to single-use containers, packaging, and vials. If part of a multiuse container is discarded, the provider may bill only for the amount that was actually administered to the patient.
CMS introduced the requirement of reporting “waste” from single-use vials on claim forms in 2017. When billing for drug waste, the provider must report the drug administered from a single-use vial on the claim as two separate charges: one claim line for the amount administered (with no modifier), and one claim line for the discarded drug amount, with modifier JW (Drug amount discarded/not administered to any patient).
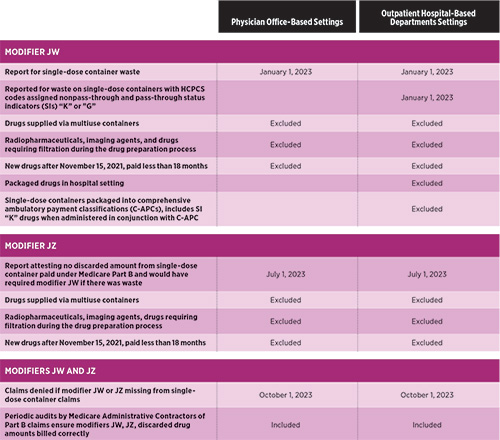
While these guidelines have been in place for several years, a new requirement is now present. The Infrastructure Investment and Jobs Act, signed into law in 2021, requires manufacturers to provide refunds for discarded amounts of refundable single-dose containers or single-use package drugs applicable above a set threshold; however, CMS understands the JW modifier is not always reported or is omitted from claims. CMS stated that the level of compliance regarding the JW modifier varied among providers, and approximately two-thirds of providers had never used the modifier. Many organizations did not comply with this requirement, so due to incomplete data related to drug discarded amounts and the impact this could have in calculating refunds by manufacturers, CMS finalized for dates of service on or after January 1, 2023, modifier JW will be required on claims for all single-dose container or single-use drugs when any amount is discarded as part of current policy and as part of this final ruling. CMS also finalized a different modifier, “JZ” (zero drug amount discarded/not administered to any patient), to attest there were no discarded amounts of a particular drug. Modifier JZ will be appended to the drug code when there is no discarded amount from the single- dose vial or single-use package, which is paid under Part B.
CMS understood providers did not have the capability to accept or report the JZ modifier immediately at the beginning delay in the requirement to use the JZ modifier to allow providers sufficient time to incorporate necessary updates to their claims systems. CMS initiated a voluntary testing period on July 1, 2023, and full implementation began October 1, 2023. If a provider cannot report the JW or JZ modifiers as required after October 1, 2023, CMS instructs that claims should be held until they are able to do so. Claims submitted without required modifier data will not be accepted and will be returned as nonprocessable.
While Medicare has published this policy regarding reimbursement for the discarded drug amounts in a single-use vial, commercial and managed care payers may have to be contacted to obtain coverage information. Insurers are not required to reimburse for the wasted drug amount in a single-dose vial, so obtaining this information in writing will assist with coding and billing compliance. It is important that you review all of the Medicare guidelines as well as your commercial payers.
To assist in outlining some of the key items, the following table provides a summary of the CMS guidelines surrounding use of modifiers for reporting single-dose container waste beginning in 2023.
While there are advocacy efforts to ease this requirement for radiology, CMS has not made any official changes to its policy, and any organization or specialty that has items that fall under these requirements must submit the JW or JZ modifiers.
Staying Keen
There are always things to pay attention to in the world of radiology coding and compliance. Keep a running list and make sure that you do not overlook anything that could impact your organization. Our world is never boring.
— Melody W. Mulaik, MSHS, CRA, RCC, RCC-IR, CPC, COC, FAHRA, is the president of Coding Strategies, Inc, and Revenue Cycle, Inc.
References
1. OfficeLinks Updates™. Aetna website. https://www.aetna.com/content/dam/aetna/pdfs/olu/officelink-updates-september-2023-olu.pdf#page6. Published September 2023.
2. NPPES FAQs. National Plan & Provider Enumeration System website. https://nppes.cms.hhs.gov/webhelp/nppeshelp/NPPES%20FAQS.html. Published 2016.

