 January/February 2026 Issue
January/February 2026 Issue
On the Case
By Isabel Okindeo, BA, and Anna Luisa Kuhn, MD, PhD
Radiology Today
Vol. 27 No. 1 P. 33
History
A 50-year-old woman presented to the emergency department with acute onset headache accompanied by nausea and vomiting. Noncontrast CT of the head and CT angiography (CTA) of the head and neck were obtained.
Findings
Coronal (Figure 1A) and sagittal (Figure 1B) maximum intensity projection images from CTA of the neck demonstrate a characteristic “string-of-beads” appearance of the right cervical internal carotid artery (ICA), consisting of alternating areas of stenosis and mild aneurysmal dilation with small outpouchings. Coronal 3D volume-rendered reconstructions (Figure 1C) further delineate the irregular vessel contour of the right ICA in contrast to the smooth appearance of the contralateral left ICA.
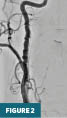
Conventional digital subtraction angiography of the right common carotid artery (Figure 2) in the frontal projection at the level of the neck confirms a long segment of alternating stenoses and dilatations involving the cervical right ICA, consistent with fibromuscular dysplasia.

Diagnosis
Fibromuscular dysplasia (FMD) of the right ICA.
Discussion
FMD is a nonatherosclerotic, noninflammatory arteriopathy characterized by abnormal arterial wall architecture. The most common manifestation consists of alternating segments of stenosis and dilatation involving small- to medium-sized arteries, producing the characteristic string-of-beads appearance. FMD may be hemodynamically significant and clinically symptomatic or may remain incidentally detected and asymptomatic.1
Historically, FMD was classified based on histopathologic findings into intimal, medial, and adventitial (periarterial) fibroplasia. As contemporary practice has moved away from surgical and histologic specimens, angiographic classification is now favored and divides FMD into multifocal and focal subtypes.
Multifocal FMD, the most common form, is characterized by collagen deposition within the arterial media and degeneration of elastic fibers, producing the classic angiographic string-of-beads appearance. In contrast, focal FMD results from fibrous tissue accumulation within the intima and manifests angiographically as short segments of tubular or concentric stenosis.2,3
FMD primarily affects women, with 85% to 91% of cases occurring in females. FMD can present at any point across the lifespan, but the mean age at the time of diagnosis is the fifth decade.4,5 FMD most commonly involves the renal arteries and the extracranial carotid and vertebral arteries, although virtually any arterial bed may be affected. Clinical presentation varies according to the vascular territory involved and most frequently includes hypertension, headache, pulsatile tinnitus, or manifestations related to aneurysm formation or arterial dissection. The exact etiology of FMD remains unknown, with limited evidence supporting a definitive hormonal or genetic cause. A dose-dependent association with cigarette smoking has been described, particularly in renal artery involvement. Because FMD is often underrecognized, its true prevalence is unknown; however, it should be considered in the differential diagnosis of women presenting with unexplained hypertension or vascular events.2,6,7
Several vascular and genetic entities should be considered in the differential diagnosis of FMD. Conditions with overlapping imaging features include atherosclerotic disease, large-vessel vasculitis, median arcuate ligament syndrome, and neurofibromatosis type 1.1 Conventional catheter angiography remains the diagnostic gold standard for FMD; however, noninvasive modalities—including duplex ultrasonography, CTA, and MR angiography—are widely used for diagnosis and follow-up. Current consensus guidelines recommend one-time brain-to-pelvis vascular imaging in all patients with FMD to evaluate for associated aneurysms, arterial dissections, and multivessel involvement. Surveillance imaging is advised for patients with known aneurysms or dissections. Management is primarily conservative and includes antihypertensive therapy and antiplatelet agents, with percutaneous transluminal angioplasty and selective surgical intervention reserved for appropriately selected cases.1,8
— Isabel Okindeo, BA, is a 3rd year medical student at the University of Massachusetts Chan Medical School in Worcester.
— Anna Luisa Kuhn, MD, PhD, is an associate professor in the division of neurointerventional radiology in the department of radiology at the University of Massachusetts Medical Center.
References
1. Gornik HL, Persu A, Adlam D, et al. First international consensus on the diagnosis and management of fibromuscular dysplasia. Vasc Med. 2019;24(2):164-189. Errata in Vasc Med. 2019;24(5):475 and 2021;26(4):NP1.
2. Narula N, Kadian-Dodov D, Olin JW. Fibromuscular dysplasia: contemporary concepts and future directions. Prog Cardiovasc Dis. 2018;60(6):580-585.
3. Krittanawong C, Kumar A, Johnson KW, et al. Prevalence, presentation, and associated conditions of patients with fibromuscular dysplasia. Am J Cardiol. 2019;123(7):1169-1172.
4. Rana MN, Al-Kindi SG. Prevalence and manifestations of diagnosed fibromuscular dysplasia by sex and race: analysis of >4500 FMD cases in the United States. Heart Lung. 2021;50(1):168-173.
5. Touzé E, Southerland AM, Boulanger M, et al. Fibromuscular dysplasia and its neurologic manifestations: a systematic review. JAMA Neurol. 2019;76(2):217-226.
6. Bhalla V, Textor SC, Beckman JA, et al. Revascularization for renovascular disease: a scientific statement from the American Heart Association. Hypertension. 2022;79(8):e128-e143.
7. Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation. 2014;129(9):1048-1078.
8. Touzé E, Southerland AM, Boulanger M, et al. Fibromuscular dysplasia and its neurologic manifestations: a systematic review. JAMA Neurol. 2019;76(2):217-226.

