 On the Case
On the Case
By Ricky Paramo, BS, and Charan Singh, MDA
Radiology Today
Vol. 24 No. 5 P. 30
History
A 64-year-old woman with a 50-packyear smoking history, COPD with chronic oxygen therapy, and a known left lung mass presented to the emergency department with a significant episode of hemoptysis and respiratory distress. The patient underwent a CT pulmonary angiogram in the emergency department.
Findings
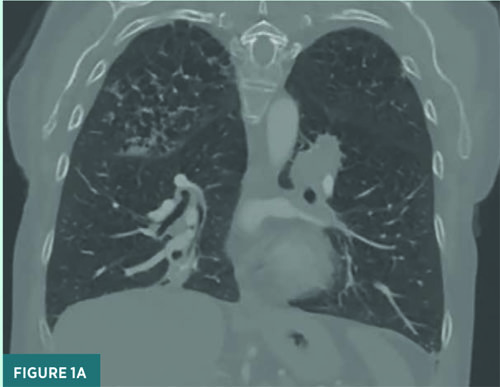
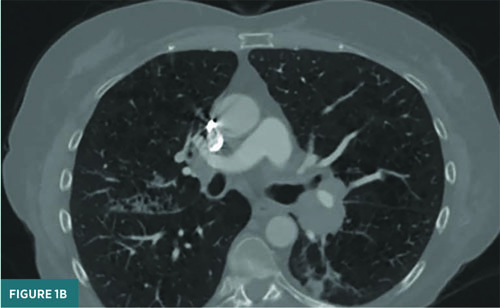
A CT pulmonary angiogram demonstrated parenchymal opacities, bronchiectasis, and left hilar lymphadenopathy (Figure 1). There was no evidence of acute pulmonary embolism. The patient was referred to interventional pulmonology and underwent bronchoscopy with biopsy. On bronchoscopy, a left endobronchial mass (causing > 90% obstruction) was biopsied. Additionally, old blood was noted throughout the tracheobronchial tree, notably on floors of the bilateral mainstem bronchi: right upper lobe and right lower lobe. These areas were lavaged and suctioned.
Initially, the patient was started on antibiotic therapy due to concern for obstructive pneumonia. In the diagnostic interim, the patient underwent bronchial artery embolization (BAE). Unique to this case, embolization was achieved via a combined approach of coiling and microspheres, where coiling prior to microsphere embolization provided a protective measure against possible spinal artery embolization.
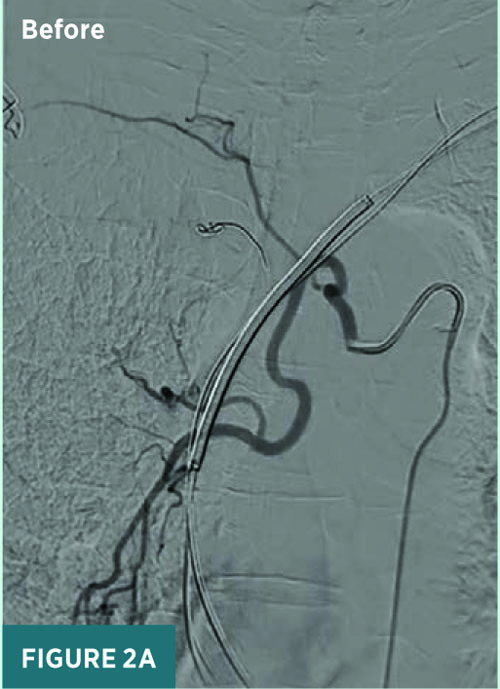
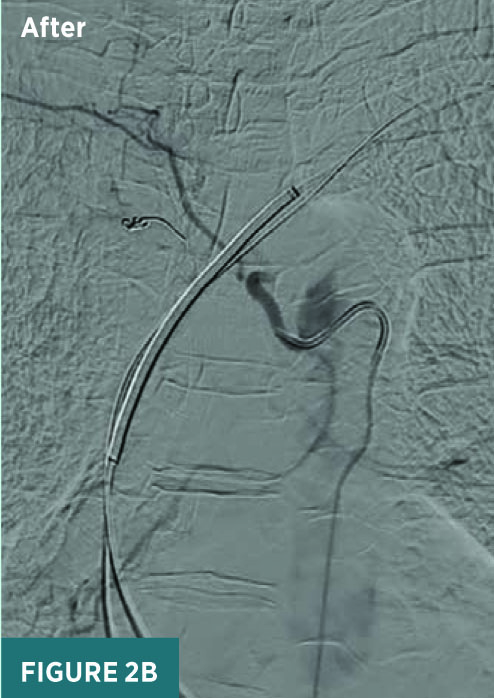
Under conscious sedation, access was achieved through the right common femoral artery for BAE. A 5 French Mickelson catheter was advanced through the thoracic aorta to perform catheterization of the right intercostobronchial trunk, which was found to be significantly hypertrophied with increased vascularity in the right perihilar and right lower lung (Figure 2). The distal right bronchial artery was then embolized using Embospheres (500 to 700 μm) and Gelfoam slurry to achieve stasis.
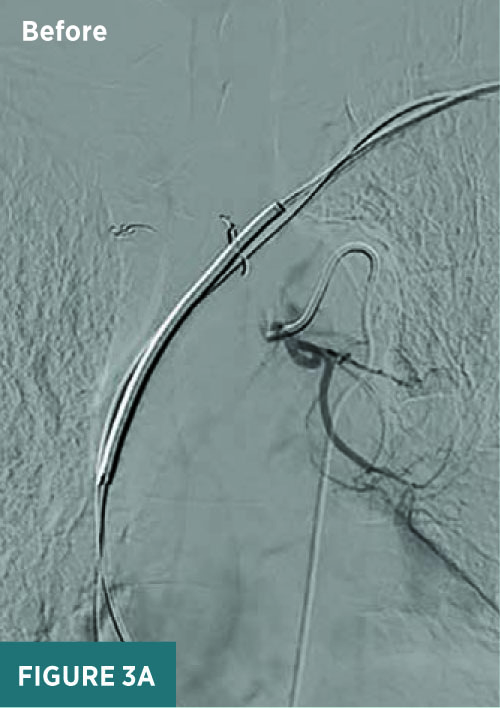
The left upper and lower intercostal bronchial trunks were also catheterized and embolized with Embospheres (500 to 700 μm) after noting hypervascularity in both perihilar and lower lung regions (Figure 3). However, prior to Embosphere use, a 5 mm coil was used to embolize the intercostal branch as a protective measure against possible spinal artery embolization.
The patient tolerated the procedure well, without complications. and achieved successful embolization of the hypertrophied bronchial arteries to address hemoptysis.

Diagnosis
Nonkeratinizing squamous cell carcinoma.
Discussion
BAE treats hemoptysis using a minimally invasive approach. Commonly used BAE materials are microspheres such as Embospheres and coils. There are benefits and risks to using each treatment.
Understanding bronchial artery anatomy is crucial for assessing risks. The right bronchial artery arises from the third posterior intercostal artery or upper left bronchial artery, while the left bronchial artery originates from the thoracic aorta or upper left bronchial artery or directly from the aorta below the level of the left subclavian artery.
At the level of the bronchial arteries, the anterior spinal artery is typically supplied by a single medullary artery from the left intercostal artery (around T4 or T5). The artery of Adamkiewicz, the largest anterior medullary branch, provides primary blood supply at lower thoracic levels (T9 to T12). Embolizing spinal arteries, including the artery of Adamkiewicz, is strongly contraindicated. Using larger particulate embolic agents (> 350 μm) helps minimize ischemic events as they cannot enter the small vessels supplying the spinal cord. However, even with larger particle sizes (> 500 to 700 μm), ischemic events cannot be eliminated, only reduced.
Microspheres are small, spherical particles that can be easily injected into small and tortuous vessels, making them useful for embolization of distal arteries or microvessels. These microspheres create a more complete occlusion of the vessel, reducing the risk of recanalization. However, they may cause embolization of unintended vessels or organs if they migrate outside the target vessel, particularly if there are anastomotic communications, leading to possible complications such as ischemia or infarction.
Coils, on the other hand, are small, flexible metal devices that can be precisely placed into the target vessel, allowing for more targeted proximal embolization of the vessel. They are relatively easy to deploy and better visualized to achieve durable occlusion of the proximal vessel. However, coils may be difficult to place in smaller or more tortuous vessels, leading to possible incomplete embolization or recanalization. Of note, if collaterals cause recurrence, previous coil embolization prevents repeated embolization of the same bronchial artery.
In some cases, using both coiling and microspheres for BAE can offer additional benefits. In this case, coiling a left intercostal branch prior to using microspheres allowed for embolization while preventing backflow or reflux of microspheres to spinal artery branches. This approach maximized the effectiveness of microsphere embolization while reducing one of its associated complications, namely ischemia or infarction. This case illustrates how a combination of different embolic agents and techniques can be used together to achieve optimal outcomes for patients undergoing BAE.
In short, BAE is a safe and effective treatment of hemoptysis, and a combination approach of embolization and coiling is a protective measure against possible spinal artery embolization. Ultimately, the choice between using microsphere particles or coils as embolic materials depends on various factors, including the anatomy of the bronchial arteries and the specific requirements of the case.
— Ricky Paramo, BS, is a medical student at the University of Connecticut School of Medicine in Farmington.
— Charan Singh, MD, is the section head of vascular and interventional radiology at UConn Health at the University of Connecticut.

