 On the Case
On the Case
By Joseph Ryan, MD, and Alex Merkulov, MD
Radiology Today
Vol. 26 No. 4 P. 30
History
A 32-year-old female, Gravida 1, Para 0, with history of sickle cell trait, presented at 41 weeks, one-day gestation for a nonstress test. Her pregnancy was complicated by a COVID infection, third trimester gestational thrombocytopenia, and a four-month gap in prenatal care during the second trimester.
A decision was made to proceed with induction of labor after the nonstress test was found to be nonreactive. She had a normal, spontaneous vaginal delivery of a 6 lbs, 13.7 oz healthy baby girl. Upon delivery of the placenta, a cord avulsion was noted and manual extraction performed. The perineum had a second-degree laceration which was repaired.
The patient’s subsequent hospital course was complicated by postpartum fevers, and she was treated with antibiotics (clindamycin, gentamicin, and ampicillin) for suspected intra-amniotic infection. Fever persisted through postpregnancy day two, and further work-up was initiated.
Initial laboratory work-up revealed thrombocytopenia with a platelet count of 93,000/uL, which had decreased from third-trimester labs six weeks earlier, with a prior platelet count of 127,000/uL. Repeat labs two days later showed downtrending platelets of 42,000/uL. Liver function tests (LFTs) were also noted to be elevated, with aspartate aminotransferase 795, alanine aminotransferase 1728, and alkaline phosphatase 306. Additional lab abnormalities included elevated total bilirubin of 2.1, elevated lactate dehydrogenase of 953. Hemoglobin nadir was 7.1, with the patient receiving one unit of packed red blood cells. An elevated reticulocytes count of 5.0% was also noted. CT of the abdomen and pelvis was then ordered.
Findings
CT imaging of the abdomen and pelvis with IV contrast revealed a 12.5 cm geographic region of hypo-enhancement in the right hepatic lobe, without associated mass effect on adjacent liver parenchyma or vasculature (Figures 1-3).

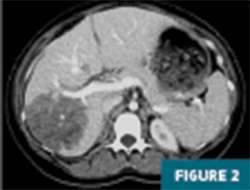
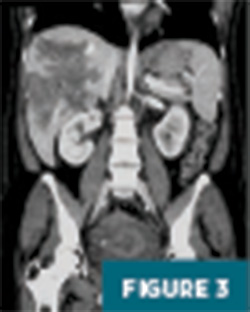
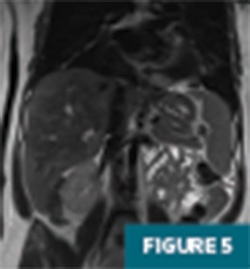
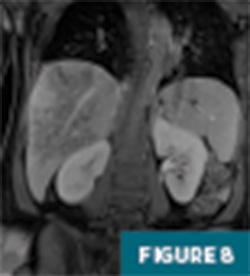
Further evaluation with MRI of the abdomen, with and without IV contrast, demonstrated a persistent large geographic area of nonenhancing hepatic parenchyma in the right hepatic lobe, with MRI morphology most consistent with an infarct (Figures 4-8). The hepatic veins and portal veins were noted to be patent on MRI. Scattered areas of T2 hyperintensity were compatible with edema within the area of presumed infarct. Reactive gallbladder wall thickening was noted without significant distension or gallstones.



Patency of the hepatic arterial and venous systems, as well as the portal veins, was subsequently confirmed on Doppler ultrasound imaging of the right upper quadrant. The region of liver infarct was also seen on ultrasound and appeared as an amorphous region of heterogeneously increased echogenicity (Figure 9).

Given the constellation of imaging findings and laboratory abnormalities, including elevated LFTs, decreased platelets, anemia, and evidence of hemolysis, the diagnosis of liver infarction due to postpartum hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome was made.
This patient was started on heparin and continued on antibiotics. She gradually showed improvement, with slow normalization of LFTs and platelets, and her fever resolved. She was discharged home on postpregnancy day six. On subsequent follow-up, she was asymptomatic. Follow-up ultrasound and MR imaging performed at two months and four months postdischarge demonstrated involution of the infarcted liver parenchyma by four months, with decreased size and conspicuity of the previously noted abnormality, mild volume loss, and posterior right hepatic lobe mild capsular retraction.
Diagnosis
Liver infarction due to postpartum HELLP syndrome
Discussion
Hepatic infarction is not a commonly encountered entity due to the liver’s dual blood supply from the hepatic artery and portal vein, with the portal vein supplying most of the blood flow to the liver parenchyma. Vascular obstruction is a major cause of a hepatic infarction; specific etiologies include postliver transplantation or hepatobiliary surgery and hepatic artery occlusion (due to arteriosclerosis, thrombosis or emboli, hepatic artery aneurysm, polyarteritis nodosa, and sickle cell). Another important subtype, infarction not caused by hepatic artery occlusion, mainly involves acute shock state/ systemic hypoperfusion, trauma, complications of anesthesia, hypercoagulable states, and pregnancy-associated pathologies (including preeclampsia, eclampsia, and postpartum HELLP syndrome).
Symptomatic patients with hepatic infarction may present with abdominal pain, nausea, vomiting, and abnormal LFTs. Coagulation panels may also help to narrow the differential. Most of the time, infarction manifests as a peripheral wedge-shaped area; however, it can also occur centrally. Oval or round shapes of infarcted parenchyma can also be seen.
On ultrasound imaging, an ill-defined hypoechoic area with indistinct margins is characteristic of an acute infarct. On ultrasound, normal structures such as veins, arteries, and bile ducts typically do not maintain their formal courses in the affected region of the parenchyma. Gas within a sterile infarcted zone can sometimes occur, making it challenging to distinguish an infarction from an abscess, sometimes necessitating fine-needle aspiration/tissue sampling. Sequela of a chronic infarct can become anechoic and cystic with more well-defined margins. Involution may also occur once some time has passed.
On CT imaging, infarction typically presents as an ill-defined, geographic, often wedge-shaped area of hypodense parenchyma, mostly peripheral without mass effect on adjacent structures on postcontrast images. On MR imaging, regions of hepatic infarction appear as hypointense lesions on T1 imaging, with heterogeneous areas of hyperintensity on T2 imaging.
General imaging differential considerations for hepatic infarction may include focal hepatic steatosis (focal fatty infiltration also lacks mass effect; however, vessels are seen crossing through lesion margins and distal/peripheral to the lesion), hepatic abscess (typically shows mass effect on adjacent structures and ring-enhancement, while hepatic infarction lacks mass effect and enhancement), true hepatic masses (both mass effect and enhancement help differentiate from infarction, and the clinical scenario is different), and emphysematous hepatitis (when gas is present, usually no mass effect).
As mentioned earlier, the underlying cause of hepatic infarction in this patient was most likely postpartum HELLP syndrome. HELLP syndrome can be thought of along the same spectrum of pathology as preeclampsia/eclampsia. Some might classify it as a severe and life-threatening form of preeclampsia, although it can occur without coexisting preeclampsia. Estimated incidence is ~0.5% of live births. The condition often occurs in the third trimester of pregnancy but can also occur postpartum, as in this case. It tends to present in young primigravid women, as was also the case here.
Clinical presentation can show variability and be characterized by a variety of nonspecific signs and symptoms. These can include malaise, epigastric and/or right upper quadrant pain, and nausea and vomiting. Some patients may have nonspecific, viral-like symptoms. Hypertension and proteinuria (classic symptoms of preeclampsia) can be extremely mild or completely absent.
General imaging/radiologic features predominantly feature hepatic sequelae, including hepatomegaly (especially of the right hepatic lobe), hemorrhage, subcapsular hematoma, rupture, and infarction. Microscopic appearance on liver histology usually includes a combination of deposited fibrin, hemorrhage, and hepatocellular necrosis surrounding portal areas. Infarction occurring in the context of HELLP syndrome could be expected to demonstrate many of these same findings and imaging patterns.
At this time, the precise etiology and pathophysiology are not fully characterized, with multiple theories posited, including immune-mediated injury (maternal acute rejection reaction to fetal antigens) and placenta-mediated liver injury (systemic inflammatory response syndrome in the setting of preeclampsia). It has been suggested that hepatic infarction and other complications associated with HELLP syndrome, including placental abruption, acute renal failure, and posterior reversible encephalopathy syndrome, may at least in part be attributed to regional vasospasm; however, this complex disease entity remains incompletely understood, and further work is needed.
This patient had several underlying conditions that may have contributed to her developing HELLP syndrome and liver infarction. COVID infection during pregnancy is one such potential factor that may have been involved, as was her underlying sickle cell trait. Her newborn’s standard genetic screening was normal. She declined more comprehensive molecular genetic testing for herself and her baby to try to uncover additional underlying predisposing risk factors that may have been implicated.
— Joseph Ryan, MD, is a radiology resident at UConn Health at the University of Connecticut in Farmington.
— Alex Merkulov, MD, is an associate professor of radiology at UConn Health.
Resources
1. Araujo ACPF, Leao MD, Nobrega MH, et al. Characteristics and treatment of hepatic rupture caused by HELLP syndrome. Am J Obstet Gynecol. 2006;195(1):129-133.
2. Escobar Vidarte MF, Montes D, Pérez A, Loaiza-Osorio S, José Nieto Calvache A. Hepatic rupture associated with preeclampsia, report of three cases and literature review. J Matern Fetal Neonatal Med. 2019;32(16):2767-2773.
3. McCormick PA, Higgins M, McCormick CA, Nolan N, Docherty JR. Hepatic infarction, hematoma, and rupture in HELLP syndrome: support for a vasospastic hypothesis. J Matern Fetal Neonatal Med. 2022;35(25):7942-7947.
4. Pavlis T, Aloizos S, Aravosita P, et al. Diagnosis and surgical management of spontaneous hepatic rupture associated with HELLP syndrome. J Surg Educ. 2009;66(3):163-167.
5. Perronne L, Dohan A, Bazeries P, et al. Hepatic involvement in HELLP syndrome: an update with emphasis on imaging features. Abdom Imaging. 2015;40(7):2839-2849.
