 On the Case
On the Case
By Arnika Karthik, MD, and Alex Merkulov, MD
Radiology Today
Vol. 26 No. 6 P. 32
History
A 28-year-old man presented for evaluation of a right distal femoral lesion incidentally discovered on imaging obtained in the emergency department (ED) following a fall. The patient slipped while exiting the shower, sustaining acute right knee pain and difficulty bearing weight.
He reported a history of chronic right knee pain for nearly a decade following a bicycle accident, as well as intermittent prodromal pain in the distal femur for over a year. At the time of his fall, he developed sudden worsening of pain and inability to ambulate, prompting emergency evaluation. Radiographs and CT imaging were obtained in the ED.
The patient reported no significant past medical history and described himself as being in good health. He denied systemic symptoms, including fevers, night sweats, unexplained weight loss, or fatigue.
On examination by orthopedic surgery, there was tenderness and swelling over the anterolateral distal thigh and knee. Range of motion of the right knee was 0°–110°, without ligamentous laxity. Hip motion was painless.
Findings
Anteroposterior and lateral radiographs of the right knee demonstrated an expansile lesion involving the lateral aspect of the distal femur. The lesion was primarily lucent and had a relatively narrow zone of transition (Figures 1 and 2). Soft tissue density involving the superior aspect of the lesion was suggestive of cortical breakthrough (Figure 1).
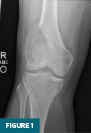
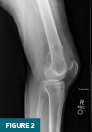
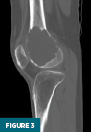
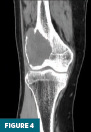
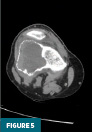
CT of the right knee without IV contrast showed a heterogenous mass within the lateral aspect of the distal femur centered in the metaphysis and extending into the epiphysis. This mass measured 7.1 cm x 6.7 cm x 6.1 cm. There was associated cortical thinning, heterogenous cortical disruption (most prominent along the supralateral aspect), and endosteal scalloping along the margins (Figures 3–5).
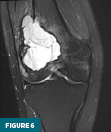
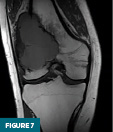
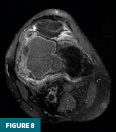
On MRI without and with IV contrast, the lesion was inhomogeneously T2 hyperintense with internal septations (Figure 6) and T1 hypointense (Figure 7). Heterogenous diffuse enhancement of the wall was noted (Figure 8). Additionally, there was moderate reactive bone marrow edema surrounding the lesion (Figure 6).
Given the constellation of findings, the top differential for this lesion included giant cell tumor, aneurysmal bone cyst, or, less likely, telangiectatic osteosarcoma. There were no findings suggestive of metastatic disease on subsequently ordered CT chest, abdomen, and pelvis imaging.
The patient was discharged from the ED and subsequently underwent outpatient orthopedic evaluation. He proceeded to open biopsy of the distal femur, performed concurrently with curettage of the lesion, adjuvant treatment, cementation of the osseous void, and prophylactic plating. Intraoperative findings were consistent with giant cell tumor of bone, and final pathology confirmed the diagnosis.
At six weeks postoperatively, the patient was ambulating primarily with a single crutch. He reported subjective instability of the right lower extremity but no significant pain at the surgical site. Range of motion was improving with ongoing outpatient physical therapy.
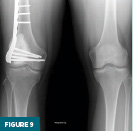
A postoperative radiograph obtained at nearly three months demonstrated the cemented defect in the distal femur with prophylactic plate and screws in appropriate alignment (Figure 9). Continued surveillance with periodic radiographs was recommended for a minimum of two years.
Diagnosis
Giant cell tumor of bone (GCTB)
Discussion
GCTB is a locally aggressive, rarely metastasizing tumor composed of neoplastic mononuclear stromal cells admixed with macrophages and osteoclast-like giant cells. It is relatively uncommon, representing 4% to 5% of all primary bone neoplasms and approximately 20% of benign bone tumors. These tumors typically occur after physeal closure, most often in early adulthood, with 80% of cases diagnosed between the ages of 20 and 50 and a peak incidence in the third decade of life. There is a slight female predominance (1.1–1.5:1). Malignant transformation occurs in fewer than 10% of cases and is more common in men (3:1). Nearly all cases of malignant GCTB are related to prior radiation therapy of an initially benign lesion.
Approximately 75% to 90% of cases arise in long bones. The distal femur is the most common site, followed by the proximal tibia and distal radius. In the axial location, followed by other vertebrae. The vertebral bodies are usually involved first, with secondary spread to the posterior elements. If disease is limited to the posterior elements, alternative diagnoses should be considered. GCTB is rarely multicentric, except in the setting of genetic syndromes such as pheochromocytomaparaganglioma and GCTB syndrome, Paget disease of bone, Gorlin-Goltz syndrome, and Jaffe-Campanacci syndrome.
Clinically, patients present with pain, swelling, and/or limited range of motion over weeks to months. Pathologic fractures may occur, resulting in an acute presentation. Neurologic symptoms can arise in patients with spinal involvement. In some cases, tumors are detected incidentally.
Definitive diagnosis relies on a combination of radiologic and histopathologic features. The differential diagnosis is broad and includes aneurysmal bone cyst (which shares overlapping features such as fluid–fluid levels), telangiectatic osteosarcoma, chondroblastoma (typically seen in skeletally immature patients), and chondrosarcoma (more common in older patients).
Radiographs, CT, and MRI all contribute to establishing the diagnosis. Radiographically, GCTB lesions are typically lytic and eccentric, arising in the metaphysis of long bones and extending into the epiphysis, often to the subarticular bone. Most lesions are geographic with a narrow transition zone. A sclerotic margin is usually absent, although up to 5% of cases demonstrate sclerosis. CT is useful for evaluating cortical thinning and breakthrough, as illustrated in this case. On MRI, lesions typically demonstrate low to intermediate signal intensity on T1-weighted images and demonstrate inhomogeneous signal intensity on T2/STIR sequences, due to hemorrhage or collagen content. Postcontrast images reveal heterogeneous enhancement. These features were evident in our patient’s imaging and were central to guiding the diagnosis.
Although not performed in this case, FDG PET usually demonstrates increased uptake and can be useful for monitoring therapy response in unresectable cases.
Management strategies depend on tumor location and patient factors. Wide resection is generally preferred to minimize recurrence. However, wide resection of a subchondral lesion often necessitates joint arthroplasty or osteochondral grafting, which is less desirable in young patients with a benign tumor. In such cases, denosumab therapy followed by marginal or wide resection may be considered. Healing lesions and metastases typically show progressive peripheral- to-central mineralization, while lack of mineralization may raise concern for misdiagnosis of primary malignant GCTB or another malignancy.
Curettage remains a common treatment option. Use of adjuvant ablative therapies in conjunction with curettage significantly lowers recurrence rates. Defects are generally filled with methyl methacrylate cement or bone graft. Cement is often favored, as bone graft may resorb prematurely and mimic tumor recurrence. Recurrence after curettage is common, reported in 15% to 50% of cases, necessitating close surveillance. Importantly, the risk of pulmonary metastasis increases in recurrent cases, despite benign histology. Radiation therapy is rarely used due to the elevated risk of malignant transformation.
In summary, GCTB is a relatively rare, locally aggressive tumor most often affecting young adults, with a slight female predominance. Although histologically benign, GCTB can undergo malignant transformation, particularly following radiation therapy. The tumor most commonly arises in long bones such as the distal femur and proximal tibia and typically presents with pain, swelling, or limited motion, though it is sometimes detected incidentally. Diagnosis relies on both imaging and pathology, with radiographs, CT, and MRI providing essential information. Treatment options include wide resection or curettage with adjuvants and cementation. Given the high recurrence rate and potential for complications, including rare metastasis and malignant transformation, close longterm follow-up is essential.
Arnika Karthik, MD, is a radiology resident at UConn Health at the University of Connecticut.
Alex Merkulov, MD, is an associate professor of radiology at UConn Health.
Resources
1. Aoude A, Nikomarov D, Perera JR, et al. Giant cell tumour of bone. Bone Joint J. 2023;105-B(5):559‑567.
2. Balke M, Ahrens H, Streitbuerger A, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol. 2009;135(1):149‑158.
3. Chakarun CJ, Forrester DM, Gottsegen CJ, Patel DB, White EA, Matcuk GR Jr. Giant cell tumor of bone: review, mimics, and new developments in treatment. Radiographics. 2013;33(1):197‑211.
4. Hosseinzadeh S, Tiwari V, De Jesus O. Giant cell tumor (osteoclastoma) [Updated January 31, 2024]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2025. https://www.ncbi.nlm.nih.gov/books/NBK559229/
5. Murphey MD, Nomikos GC, Flemming DJ, Gannon FH, Temple HT, Kransdorf MJ. From the archives of AFIP. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic‑pathologic correlation. Radiographics. 2001;21(5):1283‑1309.
6. Pereira HM, Marchiori E, Severo A. Magnetic resonance imaging aspects of giant-cell tumours of bone. J Med Imaging Radiat Oncol. 2014;58(6):674‑678.
7. Stacy GS, Peabody TD, Dixon LB. Mimics on radiography of giant cell tumor of bone. AJR Am J Roentgenol. 2003;181(6):1583‑1589.

