By Sarah E. Browning, DO
History
Three-year-old male born at full term via C-section with past medical history of global developmental delay presents with three months of worsening ataxia, headaches, and multiple episodes of emesis over the week prior to presentation. Pertinent examination findings include generalized hypotonia, slightly dysarthric speech, dysconjugate gaze, and a wide-based, ataxic gait.
Findings
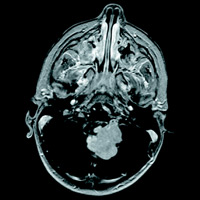
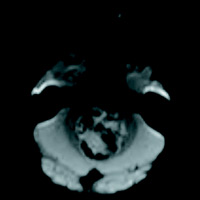
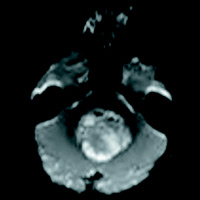
Initial head CT without contrast revealed a mostly solid mass within the fourth ventricle. The majority of the heterogeneous mass appears isointense on T1 weighted image and mildly hyperintense on T2 weighted image with multiple T2 hyperintense and T1 hypointense foci along the posterior periphery and scattered throughout related to the cystic regions of the mass. The gradient echo sequence demonstrates loss of signal anteriorly indicating blood products or mineralization. Mild restricted diffusion of the solid components is seen. With administration of contrast, diffuse hyperenhancement of the solid components and the walls of the cystic components are seen. The tumor extends into the left foramen of Luschka joints without extension into the basal cisterns. Midline sagittal imaging demonstrates mass effect on the pons and medulla with inferior herniation of the cerebellar tonsils. Obstructive hydrocephalus with T2 hyperintense signal within the periventricular white matter is suggestive of subependymal edema and impaired cerebrospinal fluid resorption. Marked thinning of the corpus callosum suggests a more chronic onset of hydrocephalus.
Diagnosis
The diagnosis was medulloblastoma. The patient underwent a suboccipital craniectomy for resection of the above mass. Histologic testing revealed a small round blue cell tumor. Initial molecular testing was negative for INI-1, which ruled out atypical teratoid rhabdoid tumor. The diagnosis was then confirmed with positive beta-catenin testing for a final diagnosis of WNT-activated, classic subtype. Postoperative MRI confirmed gross total resection with no residual tumor seen. Lumbar puncture was negative for malignant cells and MRI was negative for any leptomeningeal metastasis.
Discussion
Medulloblastomas are the most common malignant brain tumor of childhood. Seventy-seven percent of cases are diagnosed before the age of 19. Metastatic disease is present in 40% of patients at the time of diagnosis and is commonly asymptomatic. Medulloblastomas can also rarely occur in adulthood, typically during the third and fourth decades. When they do occur during adulthood, they typically carry a better prognosis.
Classically, medulloblastoma had been divided into the following long-established histological subgroups: desmoplastic/nodular, medulloblastoma with extensive nodularity, large cell, and anaplastic. More recently, however, four genetic (molecular) subgroups of medulloblastoma (WNT-activated, SHH-activated, group 3, and group 4) were identified; this called to attention the dramatic differences in prognosis and effective treatment options between the groups. This new research was included in the 2016 World Health Organization Classification of Tumors of the Central Nervous System,1 demonstrating the importance of an integrated diagnosis which contains both the histological phenotype as well as the molecular group.
Recently, researchers have begun investigating the possibility of differentiating the molecular and histological subtypes based on MRI features. Apparent diffusion coefficient value has been reported as lower in classic medulloblastoma when compared with the large cell and anaplastic types. The presence of focal cysts has also been identified in both the classic and desmoplastic subtypes. Tumor necrosis and leptomeningeal metastasis are suggestive of the large cell and anaplastic types.2 Another study found a correlation between molecular subgroups and location: WNT tumors were frequently found in the cerebellar peduncle and cerebellar-pontine angle areas, SHH tumors were seen in the cerebellar hemispheres, and group 3 and group 4 tumors were typically located in the fourth ventricle along the midline.3 MR spectroscopy has also been used to differentiate the metabolic activity of the medulloblastomas of group 3 and group 4 from the SHH subgroup.4 These preliminary studies offer an interesting noninvasive diagnostic approach for medulloblastomas.
— Sarah E. Browning, DO, is a neurosurgery resident, PGY-4, in the department of neurosurgery at Saint Barnabas Medical Center in New Jersey.
 |
 |
| Figure 1 | Figure 2 |
 |
|
| Figure 3 |
References
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
- Yeom KW, Mobley BC, Lober RM, et al. Distinctive MRI features of pediatric medulloblastoma subtypes. AJR Am J Roentgenol. 2013;200(4):895-903.
- Perreault S, Ramaswamy V, Achrol AS, et al. MRI surrogates for molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol. 2014;35(7):1263-1269.
- Blüml S, Margol AS, Sposto R, et al. Molecular subgroups of medulloblastoma identification using noninvasive magnetic resonance spectroscopy. Neuro Oncol. 2016;18(1):126-131.
Submission Instructions
- Cases should have clinical relevance and clear radiological findings.
- Seconds should include a title, history and course of illness, findings, diagnosis, and discussion.
- Word count should not exceed 800. At least three references are recommended.
- Cases may be submitted from any radiological subspecialty and imaging modality.
- Figures must be high-quality JPEG or TIFF images and labeled for ease of reference. Please keep images in their native format, without the addition of arrows or other means of highlighting the key findings.
Submit cases via e-mail to Rahul V. Pawar, MD, at rvp325@gmail.com or to Radiology Today at jknaub@gvpub.com.
Department of Radiology, Division of Neuroradiology
Saint Barnabas Medical Center/Barnabas Ambulatory Care Center