By Rahul V. Pawar, MD
History
A 47-year-old woman undergoes periodic surveillance imaging for a focal left parietal lobe lesion.
Findings
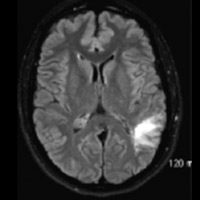
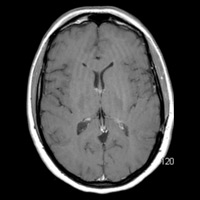
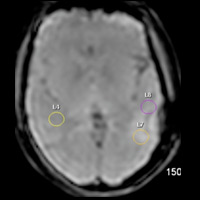
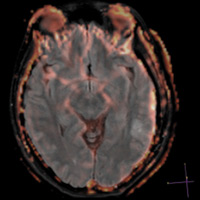
T2/FLAIR imaging demonstrates a nonenhancing, focally infiltrative hyperintense lesion within the arcuate zone of the left parietal lobe. No blooming artifact or restricted diffusion is appreciated. Morphologically, lesion has remained “stable” for seven years, exhibiting little to no mass effect. Hybrid FLAIR and relative cerebral blood volume (rCBV) map images, obtained using Philips Healthcare’s proprietary PRESTO (principles of echo shifting with a train of observations) sequence, demonstrate no significant increase in rCBV (ie, capillary density). Maximum rCBV value = 1.17.
Diagnosis
Low-grade glioma (LGG)
Discussion
• Conventional imaging of LGG typically demonstrates a relatively well-defined, homogenous mass that exhibits insignificant mass effect, minimal to no vasogenic edema, and generally no enhancement after the administration of gadolinium-based agents.1
• Unfortunately, conventional imaging features of LGG and high-grade glioma (HGG) may overlap. Determining methods differentiating between LGG and HGG is of paramount importance since treatment paradigms and prognosis are extraordinarily different.
• Although some conventional parameters have been touted for their added utility in differentiating between LGG and HGG (eg, inverse correlation between minimum apparent diffusion coefficient value and histopathologic grade of astrocytic tumors2), more reliable methods have been devised that bridge the gap between imaging and pathophysiology.
• Dynamic susceptibility contrast-enhanced (DSC) perfusion MRI is a means to this end, boosting diagnostic confidence in differentiating LGG from HGG.
• Utilizing the PRESTO sequence, a gadolinium-based agent is tracked as it passes through cerebral microvasculature. The sequence enables us to image the microscopic blood flow of gliomas. Using Philips’ Neuro T2 Perfusion Package with this patient, changes in T2 relaxation times are seen as a decrease in signal intensity that is (linearly) proportional to the local concentration of contrast that is formulated into a concentration vs. time curve. Kinetic modeling ultimately facilitates calculation of rCBV. The protocols for these exams are incorporated into Philips ExamCards, which are fully automated on either a curve fit algorithm or a deconvolution method of a predefined arterial input function. The ExamCards provide technologists with all the setting information needed to consistently perform the exams properly.
• rCBV ratios had the most superior diagnostic performance (with or without metabolite ratios) in predicting glioma grade. Sensitivity, specificity, and positive and negative predictive values for determining an HGG with conventional MR imaging were 72.5%, 65%, 86.1%, and 44.1%, respectively. Statistical analysis demonstrated a threshold value of 1.75 for rCBV to provide sensitivity, specificity, and positive and negative predictive values of 95%, 57.5%, 87%, and 79.3%, respectively.3
• In a multidisciplinary study of 35 patients with histologically diagnosed LGGs (conducted at New York University Medical Center), it was demonstrated that rCBV less than 1.75 (n = 16) had a median time to progression of 4,620 +/- 433 days, and lesions with rCBV more than 1.75 (n = 19) had a median time to progression of 245 +/- 62 days (P < 0.005). Lesions with low baseline rCBV (< 1.75) demonstrated stable tumor volumes when followed over time, and lesions with high baseline rCBV (> 1.75) demonstrated progressively increasing tumor volumes over time. This suggested that rCBV measurements may be used as a second reference standard to determine the surgical management/risk-benefit equation and postsurgical adjuvant therapy for LGGs.4
— Rahul V. Pawar, MD, is an attending radiologist with Saint Barnabas Healthcare in Livingston, New Jersey, and a consultant for Philips Healthcare North America.
 |
 |
| Figure 1 | Figure 2 |
 |
 |
| Figure 3 | Figure 4 |
- Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology. 1992;34(6):463-469.
- Lee EJ, Lee SK, Agid R, Bae JM, Keller A, Terbrugge K. Preoperative grading of presumptive low-grade astrocytomas on MR imaging: diagnostic value of minimum apparent diffusion coefficient. AJNR Am J Neuroradiol. 2008;29(10):1872-1877.
- Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24(10):1989-1998.
- Law M, Oh S, Johnson G, et al. Perfusion magnetic resonance imaging predicts patient outcome as an adjunct to histopathology: a second reference standard in the surgical and nonsurgical treatment of low-grade gliomas. Neurosurgery. 2006;58(6):1099-1107.
Submission Instructions
Submit cases directly to Rahul V. Pawar, MD, DABR (section editor for “On the Case”) at rvp325@yahoo.com. Cases submitted should be relevant and interesting. All modalities and subspecialties within radiology are equally considered.
Case submission entails two PowerPoint slides:
SLIDE 1
a. History (one-line phrase)
b. Two to five high-quality images in JPEG format without annotations
c. Name(s) of the author(s) (three maximum) and respective institutions
SLIDE 2
a. Diagnosis
b. Concise bulleted discussion (one to two lines each), including the following: pertinent clinical history, diagnostic imaging findings, differential diagnoses, treatment (if applicable)
c. Two to three relevant and current references, preferably citing peer-reviewed radiology literature
Department of Radiology, Division of Neuroradiology
Saint Barnabas Medical Center/Barnabas Ambulatory Care Center