Dreaded Complications of a Ubiquitous Pathogen
History and Course of Illness
A 20-day-old, ex-full-term male was transferred to our institution for the management of group B streptococcal (GBS) bacteremia and central nervous system (CNS) involvement. In the emergency department, the newborn was lethargic, febrile to 102˚F, and had bulging fontanelles. Cerebrospinal fluid (CSF) analysis confirmed gram-positive cocci, low glucose, and elevated protein. Antibiotics were initiated.
Within 24 hours of admission, an episode of apnea prompted emergent intubation. Postintubation, the newborn suffered clonic seizures of the left upper extremity. After urgent neurosurgical intervention and management with antiepileptics, the long-term prognosis was uncertain but likely included cortical blindness.
Findings
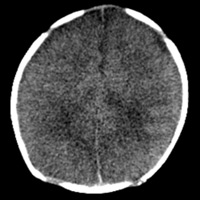
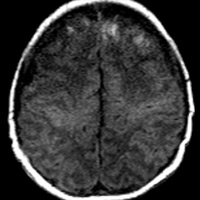
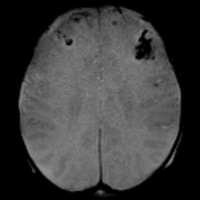
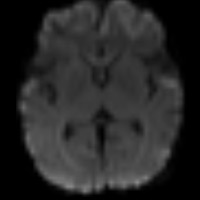
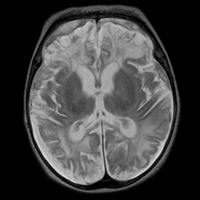
Nonenhanced CT demonstrated diffuse sulcal effacement, loss of cortico-medullary differentiation, and small bifrontal extra-axial collections (Figures A and B). T1-weighted imaging revealed the presence of bifrontal cortical and subcortical white matter hyperintensity (Figure C). The gradient-recalled echo images prominently displayed areas of blooming artifact (Figure D). Abnormal restricted diffusion was seen in a predominantly cortical distribution, affecting both hemispheres (Figures E and F). T2-weighted imaging (T2WI) (Figure G) and contrast-enhanced T1-weighted imaging (T1WI) (Figure H) showed areas of profound cortical and white matter hyperintensity, ventriculomegaly, and rim-enhancing subdural fluid collections.
Diagnosis
GBS sepsis with CT/MR evidence of diffuse cerebral edema, infarction, hemorrhage, hydrocephalus, and subdural fluid collections
Discussion
Group B streptococcus, a common pathogen of the maternal genital tract, is a well-established threat to the viability of a newborn infant. Fetal infection can occur either during gestation or during vaginal delivery. Complications include pneumonia, sepsis, and meningitis. Guidelines set forth by the Centers for Disease Control and Prevention, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics regarding the prevention of early-onset GBS were revised in 2002. Screening of all pregnant patients between 35 and 37 weeks for rectovaginal GBS colonization is mandatory; carriers are provided intrapartum antibiotic prophylaxis.1
Infected newborns may present with irritability, poor feeding, and/or lethargy; however, a subset of patients may be virtually asymptomatic. Progression to seizures and the development of raised increased intracranial pressure are late complications, signifying potential CNS damage. Although any infant may be affected, data have shown males, especially of African American descent, are at highest risk. Fortunately, screening has dramatically reduced the prevalence of disease.2
With suspected CNS involvement, the radiologist will invariably be consulted. Acutely, newborns may develop meningitis, cerebritis, vasculitis, extra-axial collections, and infarction. Ultrasound, as a first-line modality for infants, may demonstrate a nonspecific increase in parenchymal and sulcal echogenicity; dilated ventricles may be a more evident finding. Nonenhanced CT may confirm hydrocephalus and reveal hypoattenuation within white matter and/or deep gray nuclei. Subdural collections may represent reactive effusions or empyema. Multifocal hemorrhagic (venous) infarctions may be readily apparent.
MRI remains the most definitive imaging examination. Hypointense regions on T1WI represent edema and ischemia, whereas T1 hyperintensities may be a combination of hemorrhage and cortical laminar necrosis. Fluid-attenuated inversion recovery and T2 sequences depict indistinctness of gray-white differentiation and widespread hyperintense edema and pus within parenchyma and CSF spaces, respectively. Contrast-enhanced T1WI confirms meningeal, parenchymal, and ependymal enhancement. Subdural collections can be characterized further; thicker rim enhancement heralds the development of empyema. Gradient echo/susceptibility sequences exhibit evidence of hemorrhage, often not appreciated on standard T1 and T2WI. MR venography is the optimal method of detecting venous/sinus thrombosis. Last but not least, diffusion-weighted imaging will confirm areas of acute/subacute infarction with relatively high specificity.3
Recent multicenter data have emerged identifying two distinct stroke patterns observed with GBS meningitis. Hernandez et al described “deep perforator stroke” that involves periventricular white matter, basal ganglia, and thalamus as well as “superficial injury” with patchy, focal infarctions affecting the cortex (as seen in our case).4 In a prospective trial of 80 infants, a Glasgow coma scale score greater than 8, cranial nerve palsies, infarction, and hydrocephalus (confirmed by imaging) were more likely to portend severe neurological sequelae.5
Simple screening protocols are essential and failure to adhere to guidelines can be deadly. In our case, chart review uncovered a GBS-positive mother, inadequately treated, prior to normal spontaneous vaginal delivery.
— Rahul V. Pawar, MD, is a board-certified diagnostic radiologist completing a fellowship in neuroradiology at New York University Langone Medical Center.
— David H. Harter, MD, is an assistant professor of neurosurgery at New York University Langone Medical Center/NYU School of Medicine.
 |
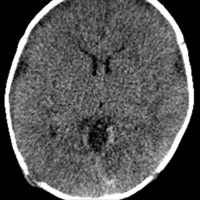 |
| Figures A and B — Nonenhanced CT demonstrates diffuse sulcal effacement, loss of cortico-medullary differentiation, and small bifrontal extra-axial collections. | |
 |
 |
| Figure C — T1-weighted imaging reveals the presence of bifrontal cortical and subcortical white matter hyperintensity. | Figure D — The gradient recalled echo images prominently display areas of blooming artifact. |
 |
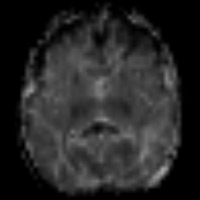 |
| Figures E and F — Abnormal restricted diffusion is seen in a predominantly cortical distribution, affecting both hemispheres. | |
 |
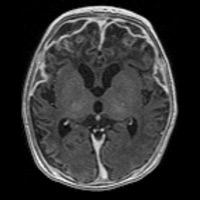 |
| Figure G — T2-weighted imaging shows areas of profound cortical and white matter hyperintensity, ventriculomegaly, and rim-enhancing subdural fluid collections. | Figure H — Contrast-enhanced T1-weighted imaging shows areas of profound cortical and white matter hyperintensity, ventriculomegaly, and rim-enhancing subdural fluid collections. |
REFERENCES
- Centers for Disease Control and Prevention. Trends in perinatal group B streptococcal disease—United States, 2000-2006. MMWR Morb Mortal Wkly Rep. 2009;58(5):109-112.
- .Hamada S, Vearncombe M, McGeer A, Shah PS. Neonatal group B streptococcal disease: Incidence, presentation, and mortality. J Matern Fetal Neonatal Med. 2008;21(1):53-57.
- Infections of the nervous system. In: Barkovich AJ (ed). Pediatric Neuroimaging. Philadelphia: Lippincott Williams & Wilkins; 2000: 715-770.
- Hernandez MI, Sandoval CC, Tapia JL, et al. Stroke patterns in neonatal group B streptococcal meningitis. Pediatr Neurol. 2011;44(4):282-288.
- Singhi P, Bansal A, Geeta P, Singhi S. Predictors of long term neurological outcome in bacterial meningitis. Indian J Pediatr. 2007;74(4):369-374.
ON THE CASE submission requirements
- Cases should have clinical relevance and clear radiological findings.
- Sections should include a title, history and course of illness, findings, diagnosis, and discussion.
- Maximum word limit should not exceed 800. At least three references are recommended.
- Cases may be submitted from any radiological subspecialty and imaging modality.
- Figures must be high-quality JPEG or TIFF images and labeled for ease of reference. Please keep images in their native format, without the addition of arrows or other means of highlighting the key findings.