By Ajay Nemade, MD, and Rahul V. Pawar, MD
History
A 78-year-old man with small-cell lung cancer presents with headaches and seizures.
Findings
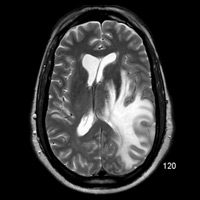
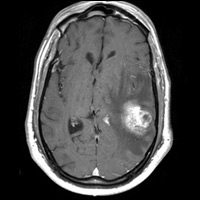
Multisequence imaging (Figure B) demonstrates an aggressive-appearing enhancing mass with the superior left temporal lobe (second lesion present within ipsilateral frontal lobe). Abundant T2 hyperintensity surrounds the lesion, effaces the left lateral ventricle, adjacent sulci, and imparts a rightward midline shift (Figure A).
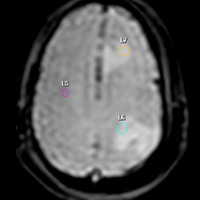
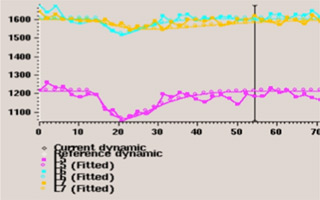
T2 images (Figure C) acquired through the PRESTO MR perfusion software package (Philips Healthcare) demonstrates three regions of interest: one within contralateral normal-appearing white matter and two others, both within edematous zones that surround enhancing lesions. rCBVmax within nonenhancing T2 signal = 0.48. The left frontal mass was a comparatively well-circumscribed ring-enhancing lesion with a diffusion negative core (not shown). Time-signal intensity curve is shown in Figure D.
Diagnosis
Metastatic lesions from lung primary. Diagnostic confidence bolstered by MR perfusion metrics.
Discussion
Enhancing lesions in the brain can represent myriad pathology. Generally, clinical history (such as in this case) is revealing. The presence of two discrete lesions also favors metastatic disease from a radiographic perspective. Yet this case illustrates the added diagnostic value of MR perfusion—it is a useful clinical tool that bolsters preoperative/pretreatment confidence.
A multidisciplinary team of specialists from Wake Forest University recently published that MR perfusion had a “significant impact on clinical decision-making and subspecialist physicians’ confidence in management plans for patients with brain tumor.”1 At the Barnabas Health Ambulatory Care Center, we routinely use a perfusion package as part of our comprehensive neuroimaging program.
Differential considerations for a ring-enhancing lesion include (but are not limited to) high-grade glioma (HGG), metastasis, abscess, and a few others, depending on circumstances. In this case, abscess was (clinically) felt to be unlikely, and the absence of a diffusion-positive core by conventional diffusion-weighted imaging was a pertinent negative. Multiplicity favored metastatic deposits, but the superior left temporal lesion was poorly demarcated and even seemed “infiltrative.” Proving both lesions were of similar (metastatic) origin was necessary.
With metastatic lesions, perilesional T2/FLAIR signal changes represent tumor-induced interstitial water accumulation owing to altered capillary permeability and blood-brain barrier breakdown. With HGG, perilesional T2/FLAIR signal changes reflect both interstitial water and tumor cells that infiltrate along capillary networks, both preexisting and new, the latter created via tumoral neoangiogenesis. Subsequent increases in local capillary density equates to increased cerebral blood volume.
Using our perfusion software (Philips’ PRESTO package), changes in T2 relaxation times are seen as a decrease in signal intensity that is (linearly) proportional to the local concentration of contrast that is formulated into a concentration vs. time curve. Kinetic modeling ultimately facilitates calculation of rCBV.
Law et al examined 51 patients with solitary brain tumor (33 HGG + 18 metastases; histology proven). Patients underwent conventional MR spectroscopy imaging and perfusion-weighted MRI. rCBVmean with HGG was 1.31 (p < 0.001). rCBVmean with metastasis was 0.39 (p < 0.001). MR spectroscopy demonstrated elevated choline/creatine (2.28 +/- 1.24) in peritumoral region with HGG but not with metastasis (p = 0.001).2
— Ajay Nemade, MD, is a radiology resident at Saint Barnabas Medical Center in Livingston, New Jersey.
— Rahul V. Pawar, MD, is an attending neuroradiologist at Saint Barnabas Medical Center, a clinical assistant professor at UMDNJ-New Jersey Medical School, and a consultant for Philips Healthcare.
 |
 |
| Figure A | Figure B |
 |
|
| Figure C | |
 |
|
| Figure D | |
- Geers CP, Simmons J, Anvery A, et al. Does MR perfusion imaging impact management decisions for patients with brain tumors? A prospective study. AJNR Am J Neuroradiol. 2012;33(3):556-562.
- Law M, Cha S, Knopp EA, Johnson G, Arnett J, Litt AW. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology. 2002;222(3):715-721.
Submission Instructions
- Cases should have clinical relevance and clear radiological findings.
- Seconds should include a title, history and course of illness, findings, diagnosis, and discussion.
- Word count should not exceed 800. At least three references are recommended.
- Cases may be submitted from any radiological subspecialty and imaging modality.
- Figures must be high-quality JPEG or TIFF images and labeled for ease of reference. Please keep images in their native format, without the addition of arrows or other means of highlighting the key findings.
Submit cases via e-mail to Rahul V. Pawar, MD, at rvp325@gmail.com or to Radiology Today at jknaub@gvpub.com.
Department of Radiology, Division of Neuroradiology
Saint Barnabas Medical Center/Barnabas Ambulatory Care Center